Exosome-Based Biopharmaceutical Development Service
Exosomes are nanoscale vesicles derived from the intracellular vesicle system, characterized by natural membrane structures and complex bioactive cargo. In recent years, exosomes have received significant attention in biopharmaceutical development. Compared to traditional synthetic carriers such as liposomes, exosomes exhibit superior biocompatibility, lower immunogenicity, and enhanced drug delivery capabilities. Exosomes can be engineered to carry specific RNA, proteins, and small molecule drugs, and they can also target specific cells through surface modifications. This makes exosomes a promising platform for clinical applications in cancer, neurodegenerative diseases, autoimmune disorders, and more.
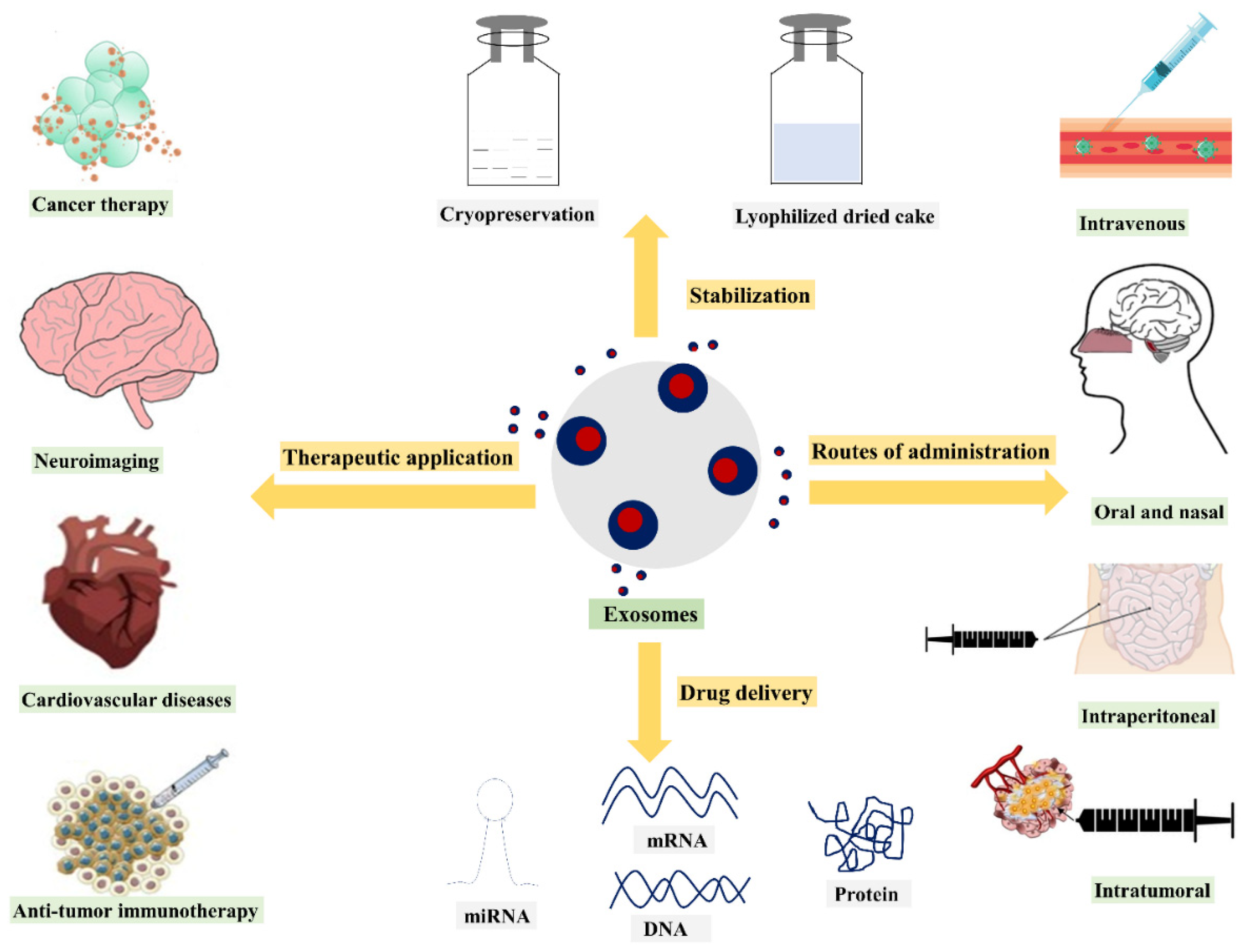
Butreddy, A. et al. Nanomaterials. 2021.
Figure 1. Overview of Exosome-Based Biopharmaceutical Strategies
MtoZ Biolabs has years of expertise in exosome research, integrating advanced mass spectrometry platforms, nanoparticle analysis technologies, and multi-omics systems to offer Exosome-Based Biopharmaceutical Development Service. The Exosome-Based Biopharmaceutical Development Service provides comprehensive solutions, including exosome drug carrier design, delivery system evaluation, and vaccine development, aimed at transforming exosomes into highly efficient and safe next-generation therapeutic tools.
Services at MtoZ Biolabs
MtoZ Biolabs focuses on exosomes as both drug delivery systems and vaccine carriers. We have built an efficient, customizable service system that supports the development of innovative biopharmaceuticals.
1. Exosomes as Drug Carriers
Using engineering modification techniques, we enable the efficient loading and stable delivery of nucleic acids (e.g., mRNA, siRNA, ASO, miRNA, circRNA), proteins, and small molecule drugs in exosomes. Targeting ligand modifications enhance selectivity for specific tissues. Whether for anti-tumor, anti-viral, or gene therapy research, MtoZ Biolabs provides stable, high-yield custom exosome carrier services.
2. Exosome-Based Vaccine Development
Exosome-based vaccines are considered a next-generation vaccine platform, offering the natural ability to deliver antigens and activate the immune system. MtoZ Biolabs provides exosome vaccine construction services, covering antigen presentation, immune modification, candidate vaccine preparation, and in vitro/in vivo functional validation. We offer customized development plans for exosome vaccines targeting infectious diseases (e.g., COVID-19, Dengue) and cancer immunotherapy, bridging the gap between laboratory research and clinical translation.
In addition to these two core services, MtoZ Biolabs has built a comprehensive platform covering exosome separation, characterization, multi-omics analysis, and functional validation, supporting various R&D needs. Specific services offered are detailed below:
|
Comprehensive Exosome Analysis Services by MtoZ Biolabs |
|||
|
Ultracentrifugation and Microfiltration based Exosome Purification |
Classical method for enriching exosomes from large-volume samples; suitable for preparative applications. |
||
|
Size Exclusion Chromatography (SEC) based Exosome Purification |
Gentle, size-based separation preserving exosome integrity; ideal for downstream functional studies. |
||
|
High-purity isolation targeting specific exosome surface markers using antibody-conjugated platforms. |
|||
|
Physical Characterization |
Measures particle size distribution and concentration; widely used for routine exosome quality assessment. |
||
|
Provides direct visualization of exosome morphology; considered the gold standard for structural validation. |
|||
|
Enables single-particle analysis of size, concentration, and zeta potential with high precision. |
|||
|
Confirms exosome identity and purity by detecting classical (e.g., CD63) and negative (e.g., Calnexin) markers. |
|||
|
Quantifies surface proteins and distinguishes subpopulations using fluorescent antibody labeling. |
|||
|
High-sensitivity single-particle analysis of exosome size, concentration, and surface marker expression. |
|||
|
Mass spectrometry-based profiling of exosomal proteins to reveal functional content and biomarkers. |
|||
|
Identifies low-molecular-weight metabolites in exosomes to explore their metabolic roles and signatures. |
|||
|
Comprehensive analysis of lipid species involved in membrane structure and signaling. |
|||
|
High-throughput sequencing of total RNA to reveal lncRNA, miRNA, and mRNA content. |
|||
|
Fluorescent or functional labeling for exosome tracking and uptake studies. |
|||
|
Custom engineering of exosomes for targeted delivery to disease-specific tissues or cells. |
|||
|
Incorporates therapeutic cargos such as RNA, proteins, or small molecules into exosomes. |
|||
|
Exosome-Associated Adeno-Associated Virus (AAV) Vector Production |
Combines exosomes with AAV vectors to enhance gene delivery and targeting efficiency. |
||
|
Assesses cellular-level effects such as proliferation, migration, and immune modulation. |
|||
|
Evaluates biodistribution and biological activity of exosomes in animal models. |
|||
Why Choose MtoZ Biolabs?
✔ High Purity Exosome Preparation System: A flexible combination of physical, immunoaffinity, and size exclusion strategies for efficient exosome extraction from various sample types.
✔ Multi-Dimensional Omics Support: Integrated analysis of proteomics, metabolomics, lipidomics, and whole transcriptomics to explore exosome functional mechanisms.
✔ High Drug Delivery Efficiency: Engineered exosomes support efficient cargo loading and targeted delivery.
✔ Strong Vaccine Immunogenicity: Achieve precise antigen presentation and efficient immune system activation.
✔ Comprehensive Platform Integration: A one-stop platform combining exosome separation, characterization, engineering, and functional validation.
✔ Customizable R&D Routes: Flexible services tailored to different targets, drug types, and research stages.
Sample Submission Suggestions
We accept a variety of biological samples (e.g., cell culture supernatants, serum, plasma, urine, cerebrospinal fluid), as well as purified exosomes. Please contact us for specific sample preparation and shipping guidelines to ensure successful experimentation.
What Could be Included in the Report?
1. Exosome Purity and Marker Identification Report
2. Cargo Loading Efficiency and Modification Validation Data
3. Omics Analysis Results (Proteomics, Transcriptomics, Metabolomics)
4. Targeting/Release Characteristics Functional Validation Report (if applicable)
5. Complete Data Report and Raw Spectra
FAQ
Q1: What types of therapeutic substances can exosomes deliver?
Exosomes can carry and deliver various bioactive substances, including siRNA, miRNA, mRNA, ASO, proteins, and small molecule compounds, making them suitable for a variety of disease treatment models.
Q2: How can antigen presentation be ensured during exosome vaccine development?
We use genetic engineering methods to anchor target antigens to exosome membrane proteins for precise display and validate antigen expression using flow cytometry, Western blot, and electron microscopy. These strategies enhance exosome-mediated activation of dendritic cells and T-cells, improving vaccine efficacy.
Exosomes, with their low immunogenicity, high biocompatibility, and excellent drug delivery capabilities, have become a hot topic in the research of novel drug carriers and vaccine platforms. MtoZ Biolabs, with its advanced mass spectrometry platforms and exosome engineering capabilities, offers a one-stop service covering exosome separation, modification, omics analysis, and functional validation. Our Exosome-Based Biopharmaceutical Development Service provides efficient support for drug development and vaccine projects, helping you accelerate the achievement of preclinical translation goals. Please contact us for professional and customized service solutions.
How to order?







