Exosome In Vitro Functional Research Services
- Investigate the role of exosomes in tumor initiation, metastasis, and drug resistance;
- Elucidate signaling pathways and molecular networks regulated by exosomes in neurological, cardiovascular, and metabolic diseases;
- Explore how exosomes modulate immune cell function and contribute to autoimmune disease progression.
- Evaluate the functional effects of candidate exosome-based therapeutics (natural, engineered, or cargo-loaded);
- Validate the delivery efficiency and biological activity of nucleic acids, proteins, or small-molecule drugs encapsulated in exosomes;
- Screen for exosome sources or modifications that enhance tissue targeting and therapeutic performance.
- Compare the effects of different labeling, fusion proteins, or cargo-loading strategies on exosome function;
- Quantitatively assess uptake, signal pathway activation, and phenotypic regulation before and after engineering;
- Provide foundational in vitro data for in vivo validation and translational development.
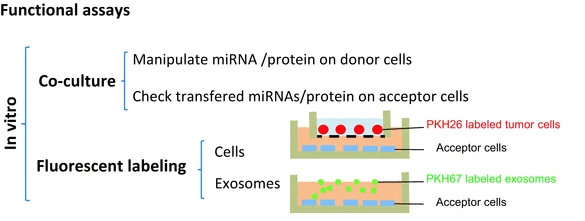
Exosome In Vitro Functional Research Services are systematic research offerings focused on analyzing the functional behavior of exosomes at the cellular level. By co-culturing exosomes with target cells and incorporating evaluation methods such as cellular uptake analysis, phenotypic changes, signal pathway activation, and molecular expression profiling, this service aims to elucidate how exosomes influence cellular behavior—including migration, apoptosis, proliferation, and immune activation or suppression.
Exosomes can be internalized by target cells through receptor binding, endocytosis, or membrane fusion, subsequently releasing their bioactive cargo intracellularly to modulate downstream signaling pathways and alter cellular states. To track and quantify the uptake efficiency and functional outcomes of exosomes, techniques such as fluorescent labeling, genetic engineering, or functional reporter systems are commonly employed for visualization and quantitative analysis. During functional validation, co-culture of exosomes with recipient cells allows researchers to assess their regulatory effects through indicators like cell proliferation, apoptosis, and migratory capacity, providing direct experimental evidence for exosome-mediated functional mechanisms.
Valencia K. Lecanda F. Springer New York. 2016.
Leveraging a well-established cell-based experimental platform, MtoZ Biolabs offers Exosome In Vitro Functional Research Services to comprehensively evaluate the impact of exosomes on key cellular behaviors such as proliferation, migration, invasion, and apoptosis. Through various labeling techniques and co-culture strategies, this service enables researchers to investigate the functional roles of exosomes at both the molecular and cellular levels, providing essential data to support basic research and clinical translational applications.
Technical Principles
Exosome In Vitro Functional Research Services offered by MtoZ Biolabs are grounded in the following core principles:
Validation of Cellular Uptake Mechanisms
Exosomes are actively internalized by recipient cells via endocytosis, membrane fusion, or receptor-mediated uptake. Their membrane protein composition and surface modifications determine targeting specificity. Using fluorescent dyes (e.g., DiR, PKH26), combined with confocal microscopy and flow cytometry, we track the uptake kinetics and intracellular localization of exosomes in various cell types.
Functional Molecule Transfer
Once internalized, exosomes can deliver functional cargos such as miRNAs, lncRNAs, and proteins to recipient cells, modulating gene expression and cellular function. Techniques like RT-qPCR and Western blotting are employed to assess target molecule changes and evaluate the regulatory efficacy of exosomal cargo delivery.
Regulation of Cellular Behavior
Exosomes influence recipient cell phenotypes by modulating signaling pathways such as PI3K/AKT, MAPK, and TGF-β. These effects can alter cell proliferation, migration, apoptosis, and inflammatory factor expression. In vitro models are used to systematically observe and quantify these biological effects.
Applications
MtoZ Biolabs’ Exosome In Vitro Functional Research Services are applicable to a wide range of research and development fields:
1. Disease Mechanism Studies
2. Therapeutic Exosome Development
3. Engineering Optimization of Exosomes
FAQ
Q. Can your Labeling Methods Distinguish between Membrane Fusion and Endocytic Uptake Mechanisms?
Yes. We employ differential dye strategies combined with confocal microscopy and flow cytometry to preliminarily distinguish these pathways. Lipophilic dyes (e.g., PKH26) trace membrane fusion, while intracellular-activation dyes (e.g., CFSE) track endocytosis.
Q. How to Ensure that Exosome Labeling does not Alter Biological Activity?
We perform rigorous pre- and post-labeling validation, including nanoparticle tracking analysis (NTA), membrane marker expression (CD63/CD81), uptake ability, and cellular responses to ensure labeling does not compromise exosome bioactivity.
Q. How do you Control for Dye Residue or Nonspecific Binding?
We use column chromatography or ultrafiltration to remove unbound dye and include “dye-only controls” to assess background signal from nonspecific adsorption, ensuring that observed effects are truly exosome-mediated.
Case Study
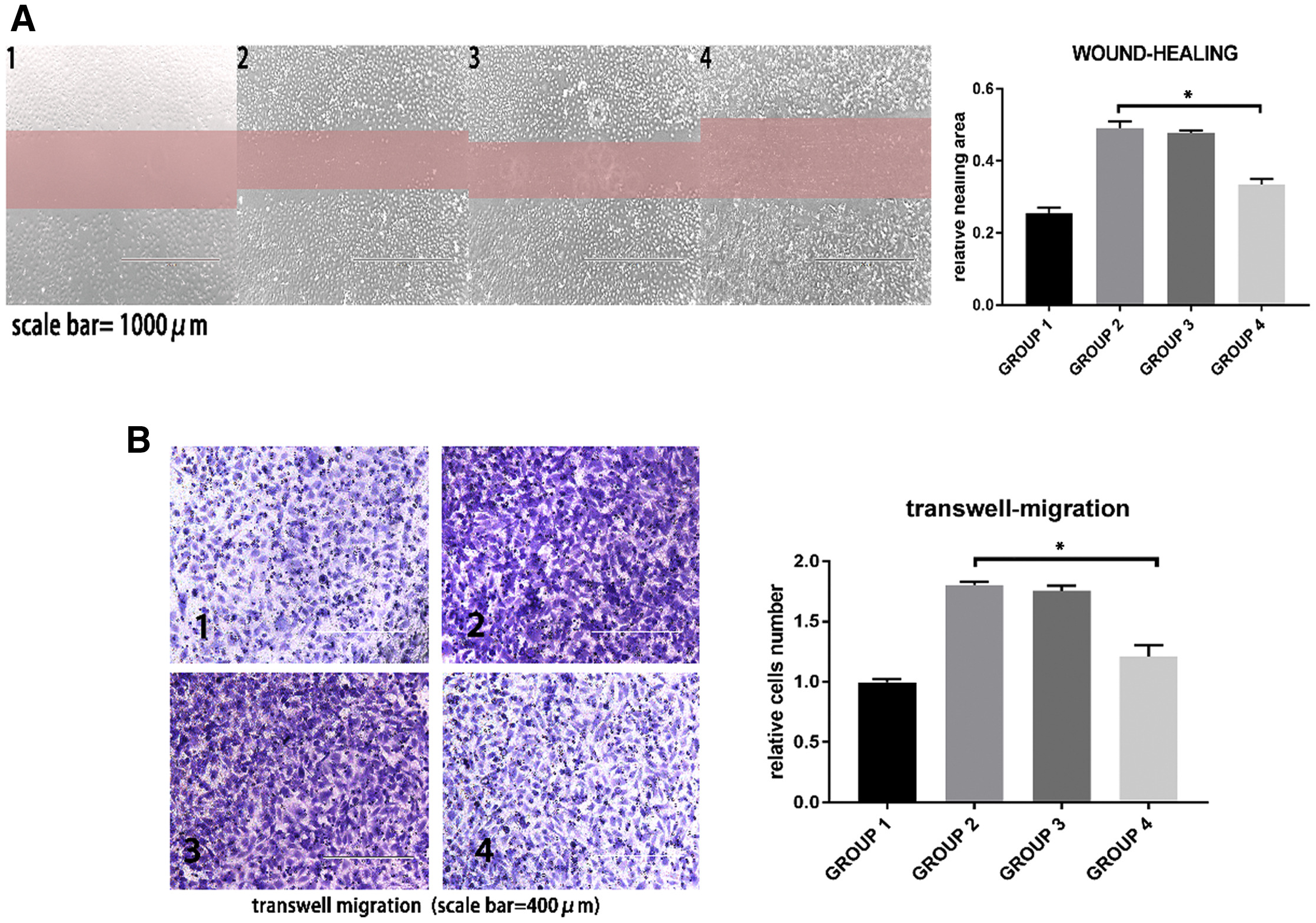
In this study, multiple in vitro functional assays were used to evaluate the anti-atherosclerotic effects of mesenchymal stem cell (MSC)-derived exosomal miR-145. Fluorescent labeling confirmed the uptake of exosomes by human umbilical vein endothelial cells (HUVECs). Transfection, qRT-PCR, and Western blotting demonstrated that miR-145 delivered via exosomes suppressed JAM-A expression. Functional assays, including migration analysis, revealed that miR-145-enriched exosomes significantly inhibited HUVEC migration. Additional assays for endothelial permeability and immunofluorescence confirmed that exosome-mediated delivery of miR-145 effectively alleviated JAM-A–dependent barrier dysfunction.
Yang W. et al. Mol Ther Nucleic Acids. 2021.
How to order?







