Vaccine Quality Research and Testing Solutions
Vaccines are biological products used to prevent infectious diseases. They stimulate the human immune system by mimicking infections caused by pathogens such as viruses or bacteria. Vaccines typically contain weakened or dead forms of the pathogen, parts of the pathogen (such as proteins or polysaccharides), or just the genetic material of the pathogen. These components are sufficient to enable the immune system to recognize them but do not cause the disease.
When a vaccine is injected or taken orally, it prompts the immune system to produce a defensive response against the pathogen, including the production of antibodies and the activation of immune cells. This process leaves an "immune memory," enabling the immune system to quickly recognize and eliminate the same pathogen in the future, thereby preventing the occurrence of the disease or mitigating its severity.
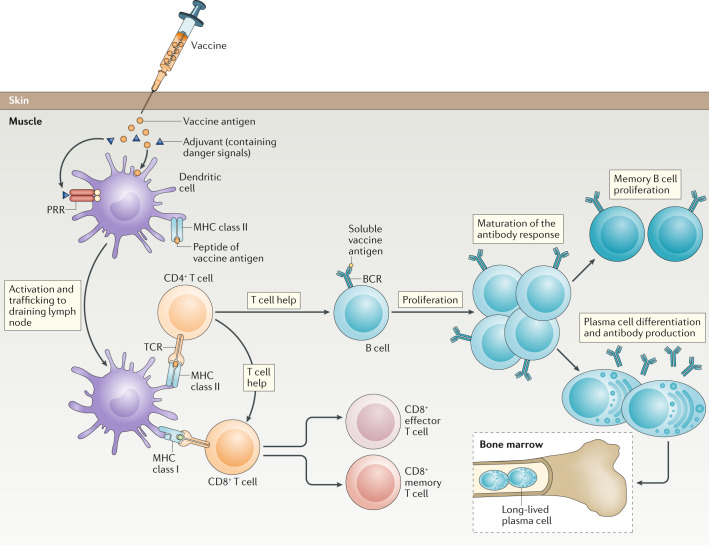
Figure 1. Vaccine-Induced Immune Response[1]
Classification and Characteristics of Vaccines
1. Live-Attenuated Vaccines
These vaccines use weakened or non-toxic live microbial pathogens. They can grow and reproduce within the body, thus producing a strong immune response, and usually only require a single dose. Representative drugs include the bacillus Calmette-Guérin (BCG) vaccine, cowpox vaccine, measles vaccine, and oral polio vaccine (OPV).
2. Inactivated Vaccines
Made from killed microbial pathogens, these cannot reproduce within the body, thus multiple doses are required to produce a lasting immune effect. Inactivated vaccines are usually safer but may need booster shots. Representative drugs include hepatitis B vaccines (such as certain types of hepatitis B vaccines), split influenza vaccines, and COVID-19 inactivated vaccines.
3. Subunit Vaccines
These vaccines contain only specific components of the pathogen, such as proteins or polysaccharides, sufficient to stimulate an immune response. Subunit vaccines generally have fewer side effects but may require adjuvants to enhance immunogenicity. Representative drugs include hepatitis B subunit vaccine and acellular pertussis subunit vaccine.
4. Toxoid Vaccines
By chemically treating the toxins of pathogens to remove their toxicity while retaining immunogenicity, used to prevent diseases caused by exotoxins, such as tetanus and diphtheria vaccines.
5. Vector Vaccines
Use a harmless virus or bacterium as a carrier that carries the gene of the target pathogen. After entering the host cells, it produces specific antigens, thereby stimulating an immune response.
6. Nucleic Acid Vaccines
Include DNA vaccines and RNA vaccines, which involve introducing exogenous genes that encode antigenic proteins directly into host cells, where they are synthesized by the host cell's expression system to induce an immune response. Representative drugs include certain types of hepatitis B vaccines, and nucleic acid vaccines (including DNA and mRNA vaccines).
7. Conjugate Vaccines
Polysaccharide antigens are combined with proteins to enhance the immunogenicity of the vaccine, especially effective in infants and young children. For example, the meningococcal polysaccharide-protein conjugate vaccine.
8. Recombinant Protein Vaccines
Using genetic engineering techniques, microorganisms or cells are made to produce specific proteins of the pathogen, which are used as antigens in the vaccine.
9. Transgenic Plant Vaccines
Antigen genes are introduced into plants, and immunity is obtained by consuming these plants or parts of them.
10. mRNA Vaccines
Use the pathogen's mRNA to induce the body to produce viral proteins, thereby triggering an immune response, such as the Pfizer-BioNTech COVID-19 vaccine (BNT162b2), and the Moderna COVID-19 vaccine (mRNA-1273).
Advantages and Limitations of Vaccines as Therapeutic Drugs
1. Advantages
(1) Significant Preventive Effect
Vaccines can effectively prevent the occurrence of various infectious diseases, reduce the spread of diseases, and lower incidence and mortality rates.
(2) Immune Memory
Vaccines can form long-term immune memory in the human body. When the same pathogen is encountered again, the immune system can quickly respond and effectively resist infection.
(3) Herd Immunity
When a sufficient number of the population is vaccinated, herd immunity can be formed, reducing the spread of disease in society, even if some individuals are not vaccinated.
(4) Cost-Effective
Compared to treating diseases, vaccines usually cost less, and preventing diseases can reduce the consumption of medical resources and the economic burden on families.
(5) Reduced Antibiotic Use
By preventing bacterial infections, vaccines help reduce the use of antibiotics, thereby slowing the development of antibiotic resistance.
2. Limitations
(1) Long Development Cycle
The development and approval process for vaccines usually takes a long time, which may be insufficient in response to sudden public health events.
(2) Safety Issues
Although the safety of vaccines is rigorously tested, adverse reactions may still occur, especially in individuals with weak immunity.
(3) Vaccination Coverage
The effectiveness of vaccines is affected by vaccination coverage; if the vaccination rate is insufficient, effective herd immunity may not be formed, reducing the effectiveness of the vaccine in preventing diseases.
(4) Mutating Pathogens
Variants of pathogens may affect the effectiveness of vaccines, necessitating continual updates to vaccines to address new strains.
(5) Public Trust
Public trust in vaccination has a significant impact on vaccination rates. Misunderstandings and rumors can lead to decreased vaccination rates, affecting the effectiveness of vaccines in preventing diseases.
(6) Specific Population Restrictions
Some vaccines may not be suitable for specific populations, such as pregnant women or patients with compromised immune systems, limiting the general applicability of vaccines.
Strategies to Overcome Limitations Associated with Vaccines
1. Improved Vaccine Design
Through the use of reverse vaccinology and immunoinformatics approaches, identify conserved and highly immunogenic epitopes, and develop multiepitope vaccines, such as recombinant protein, DNA, or mRNA vaccines.
2. Optimization of Vaccine Formulation
Research and develop new antigens and vaccine formulations to effectively stimulate immune responses, and enhance the stability and safety of vaccines.
3. Enhanced Vaccination Strategies
Adjust the target populations for priority vaccination based on the dynamics of the epidemic to effectively utilize the direct and indirect protective effects of vaccines, while controlling the number of infections and deaths.
4. Increased Thermal Stability of Vaccines
Develop dry vaccines, and use appropriate drying techniques such as spray drying, freeze drying, and spray freeze drying, to enhance vaccine stability and reduce dependence on cold chain logistics.
5. Improved Storage and Transportation of Vaccines
Study the use of stabilizers to protect vaccines from adverse conditions, enabling vaccines to be stored under environmental conditions and facilitating transportation to warm climates and developing countries.
6. Sequential Immunization
Employ mixed vaccination using different vaccines to achieve effective protection and sustained immunity that cannot be achieved by a single type of vaccine.
7. Utilization of Novel Vaccine Platforms
Develop next-generation vaccine platforms, such as mRNA vaccines, to rapidly adapt to viral mutations and accelerate the development and production of vaccines.
8. Strengthened Clinical Research
Conduct more preclinical and clinical studies to assess the safety, immunogenicity, and efficacy of vaccines, especially in the face of rapidly mutating pathogens.
9. Increased Public Awareness and Trust
Through promotional campaigns, enhance the willingness to vaccinate among targeted populations, particularly among the elderly and those with underlying diseases.
10. Continuous Monitoring and Assessment
Continuously monitor and assess the long-term effects of vaccination to promptly identify and address potential issues.
Testing Content Required by Policies and Regulations
Guidance for Industry Content and Format of Chemistry, Manufacturing and Controls Information and Establishment Description Information for a Vaccine or Related Product
1. Assessment Criteria and Testing Methods for Drug Substance
(1) Description
① Biological Name: Includes strain or clone names.
② Chemical Name: Includes established common name (USAN).
③ Source: The origin of cells, including microbes, viruses, or parasites.
④ Physicochemical Properties: The physical and chemical properties of the synthetic drug substance.
⑤ Chemical Modification or Conjugation: Detailed description of any chemical modifications or conjugations.
(2) Characteristic Testing
① Composition: Analyzed using ultraviolet/visible (UV/Vis) spectroscopy, mass Spectrometry (MS), etc.
② Purity: Through amino acid analysis, carbohydrate analysis, peptide mapping, SDS-PAGE, isoelectric focusing (IEF), etc.
③ Potency: Assessed by bioassays such as specific identity tests, cytometric assays, neutralization tests, etc.
④ Stability: Assessed through appropriate analytical methods to evaluate the stability of the drug substance.
(3) Production Process Control
① Raw Materials: Specifications and tests for culture media, buffers, synthetic resins, chemicals, etc.
② Process Diagram: Shows the production steps, materials used, and equipment involved.
③ Detailed Description: Includes detailed descriptions of animal-derived, virus-derived, and cell-derived sources.
(4) Process Control
① Intermediate Process Control: Tests conducted at critical stages of production.
② Process Validation: Validation of key processes or factors affecting the specifications of the drug substance.
(5) Specifications for Drug Substance
① Specifications: Includes tests for identity, purity, potency, physicochemical measurements, and stability.
② Impurity Profile: Includes identification and quantification of product-related and process-related impurities.
2. Assessment Criteria and Testing Methods for Drug Product
(1) Composition and Characteristics
① Drug Substance: Lists all drug substances.
② Excipients: Lists all inactive ingredients and explains their role in the final product.
③ Adjuvants: Lists the chemical formulas and exact amounts of each adjuvant.
④ Preservatives: Identifies each preservative and provides results of preservative efficacy studies.
(2) Specifications and Analytical Methods
① Description: Provides a description of the physical state, color, and clarity of the drug product and components.
② Identity: Describes the analytical methods used to determine the identity of the drug product.
③ Purity and Impurities: Provides information on the final product's purity, including identification and quantification of impurities.
④ Potency: Describes the methods for determining the potency of the drug product.
(3) Manufacturing Methods
Process: Includes detailed descriptions of formulation, aseptic processing, lyophilization, and packaging.
(4) Specifications for Drug Product
① Sampling Procedure: Monitoring procedures for finished product batches.
② Specifications and Methods: Selection of test methods and specifications to ensure the finished product's identity, purity, strength, and/or potency.
(5) Containers and Closure Systems
Compatibility: Describes the compatibility of containers and closure systems.
(6) Microbiology
Sterility: Verification of the aseptic processing.
(7) Lyophilization
Validation: Provides a summary of the validation of the drug product's lyophilization.
(8) Stability
① Stability Protocol: Includes tests for potency, physicochemical measurements, humidity, pH, sterility, or bioburden control, etc.
② Stability Data: Provides data supporting the proposed shelf life and recommended storage conditions.
GUIDANCE FOR INDUSTRY FOR THE EVALUATION OF COMBINATION VACCINES FOR PREVENTABLE DISEASES: PRODUCTION, TESTING AND CLINICAL STUDIES
1. Testing Criteria for Production and Testing Phases
(1) Component Compatibility: Ensure compatibility among the components in the combination vaccine to ensure safety and effectiveness.
(2) Preservatives: Evaluate the impact of using preservatives in the combination vaccine, including their potential effects on the potency of other components.
(3) Adjuvants: Assess the safety and immunoenhancing effects of adjuvants.
(4) Inactive Ingredients: Evaluate the effects of different buffers, salts, and other chemicals on the safety, purity, and potency of the final combination vaccine.
(5) Stability/Shelf Life: Provide stability data for the combination vaccine to support its shelf life.
(6) Production Consistency: Demonstrate consistency in the manufacturing process by producing a certain number of batches.
2. Testing Criteria for Preclinical and Clinical Study Phases
(1) Safety: Assess the safety of the combination vaccine through clinical studies.
(2) Immunogenicity: Evaluate the immune response induced by the combination vaccine in humans, including antibody levels, classes, affinity, half-life, and functionality.
(3) Efficacy: Demonstrate the efficacy of each component through clinical studies.
(4) Statistical Considerations: Include determination of sample size, data analysis methods, and data formats submitted to the CBER.
3. Testing Methods
(1) Identity Testing: Perform identity testing on the vaccine in the labeled final container to ensure it is consistent with the product specified on the product label.
(2) Sterility: All components of the combination vaccine and the final combination product must meet the sterility requirements outlined in 21 CFR 610.12.
(3) Purity: Each bulk component of the combination vaccine should meet the appropriate purity characteristics for that component.
4. Clinical Study Design
(1) Randomization and Control: Clinical studies should be randomized and controlled.
(2) Comparative Studies: Typically, the control group will receive the individual components of the combination vaccine simultaneously.
(3) Safety Evaluation: Proactive and prospective planning for follow-up safety assessments.
(4) Immunogenicity Studies: Study the immunogenicity of all vaccine components and compare it to individual vaccines given simultaneously but separately.
Vaccine Drug Analysis Solutions
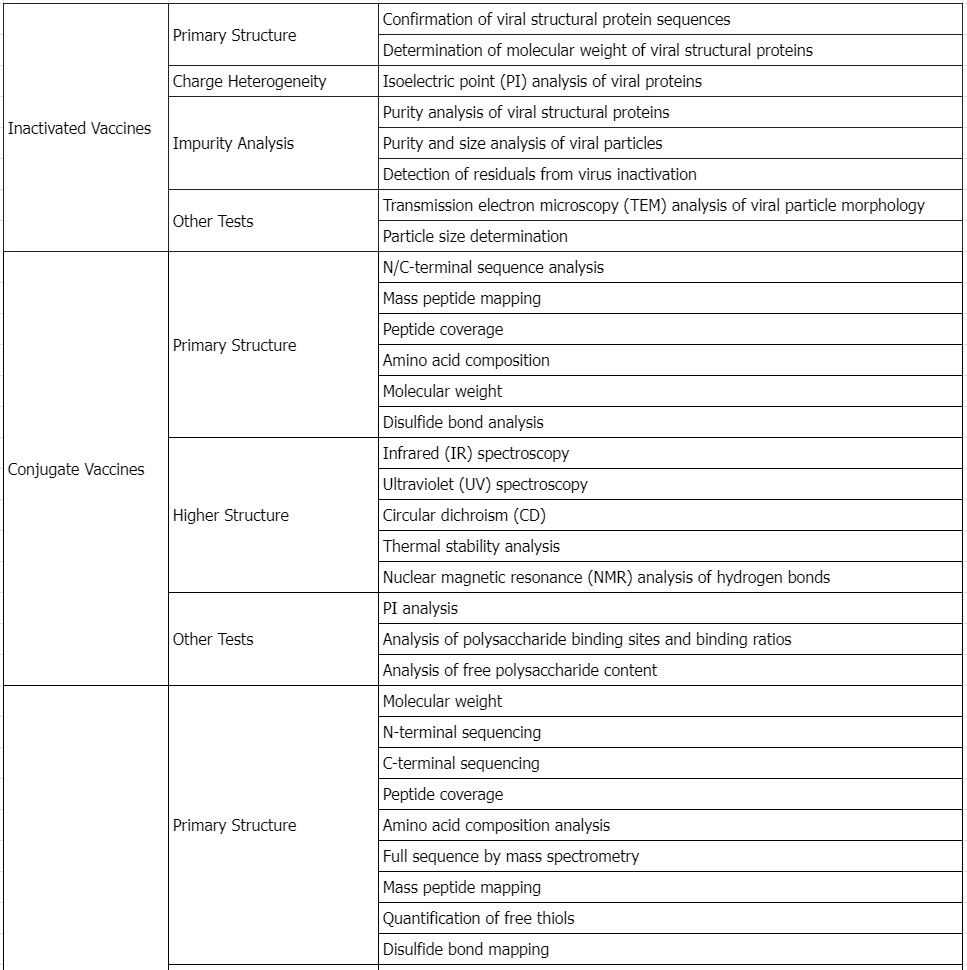
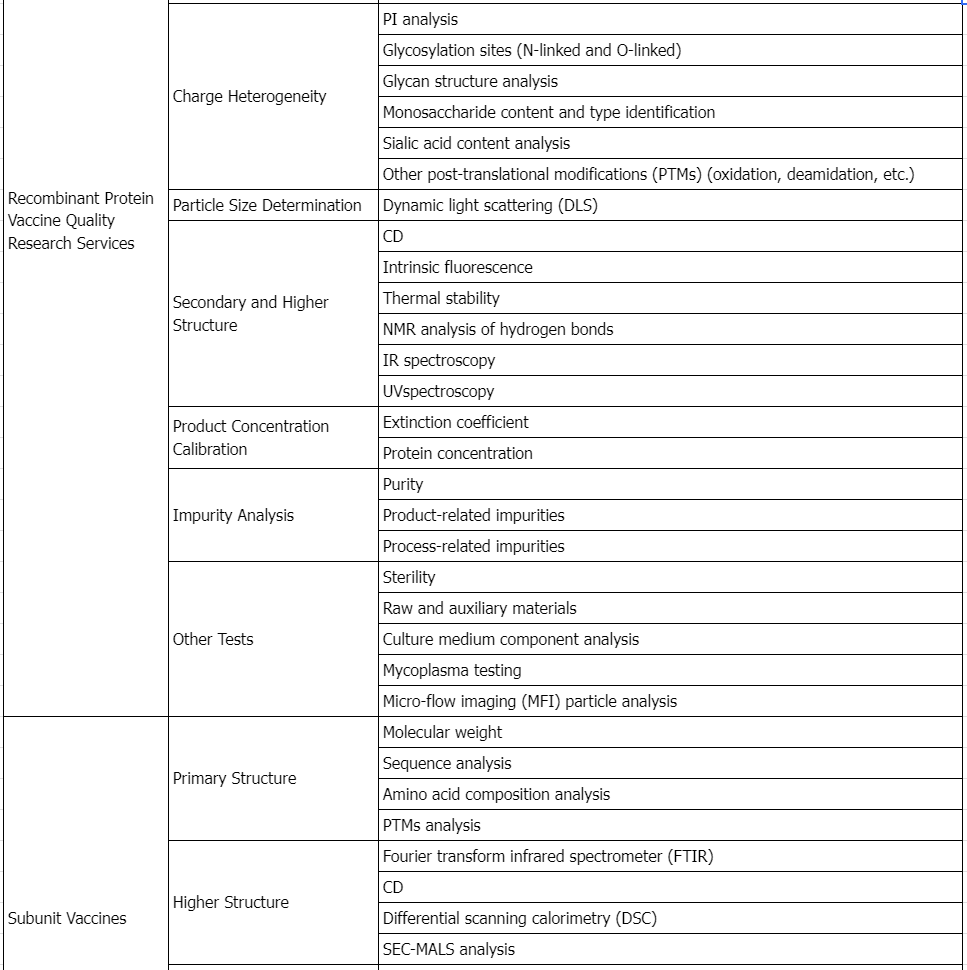
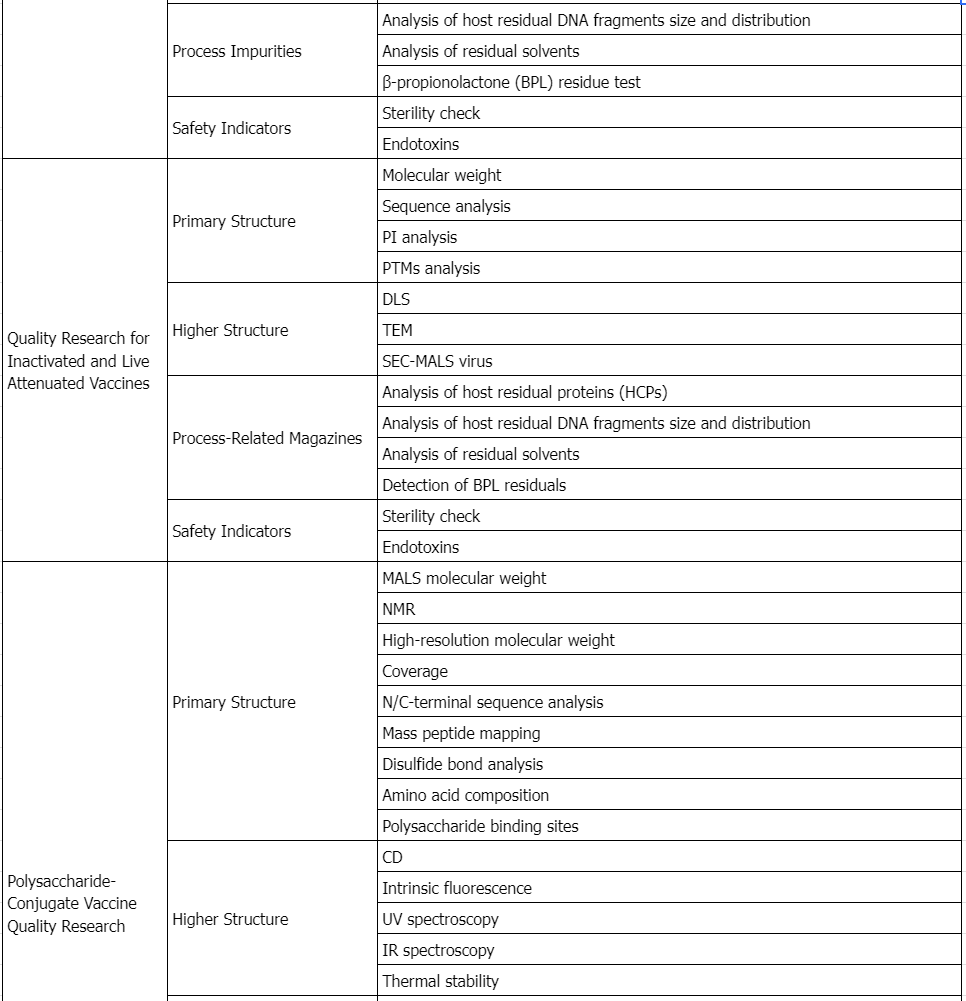
Service Advantages
1. Professional and Robust Services Covering Multiple Aspects of Vaccine Drug Testing
2. High Cost-Effectiveness, Short Experimental Cycles, and Complete and Credible Reports
3. Equipped with Various Large-Scale Instruments, Including Multiple High-Resolution Mass Spectrometers
Case Study
1. Physical Characterization and Formulation Development of a Recombinant Pneumolysoid Protein-Based Pneumococcal Vaccine
Streptococcus pneumoniae is a leading cause of death in children worldwide. There are over 90 known serotypes of pneumococcus, which vary geographically. Pneumolysin is a protein toxin produced by nearly all invasive strains of Streptococcus pneumoniae and is considered a significant virulence factor. Pneumolysin is immunogenic and has the potential to serve as a new vaccine antigen providing broad serotype-independent coverage. To develop a stable vaccine formulation, the conformational stability of a recombinant pneumolysin mutant (pneumolysoid L460D) was characterized using various techniques. Three data visualization graphs were constructed, summarizing the biophysical data of L460D pneumolysoid; the protein is most stable in solutions at pH 6-7 and loses conformational integrity above 48°C. Excipient screening experiments showed that sugars such as trehalose and sucrose stabilized the pneumolysin mutant, increasing the thermal transition temperature and minimizing aggregation. Additionally, the protein antigen's binding to aluminum hydroxide adjuvant was effective. The conformational stability of L460D pneumolysoid on the surface of the alhydrogel adjuvant was not significantly affected by adsorption, regardless of the presence of excipients. These studies provided important pre-formulation characterization information for the development of a stable pneumolysin mutant-based vaccine.
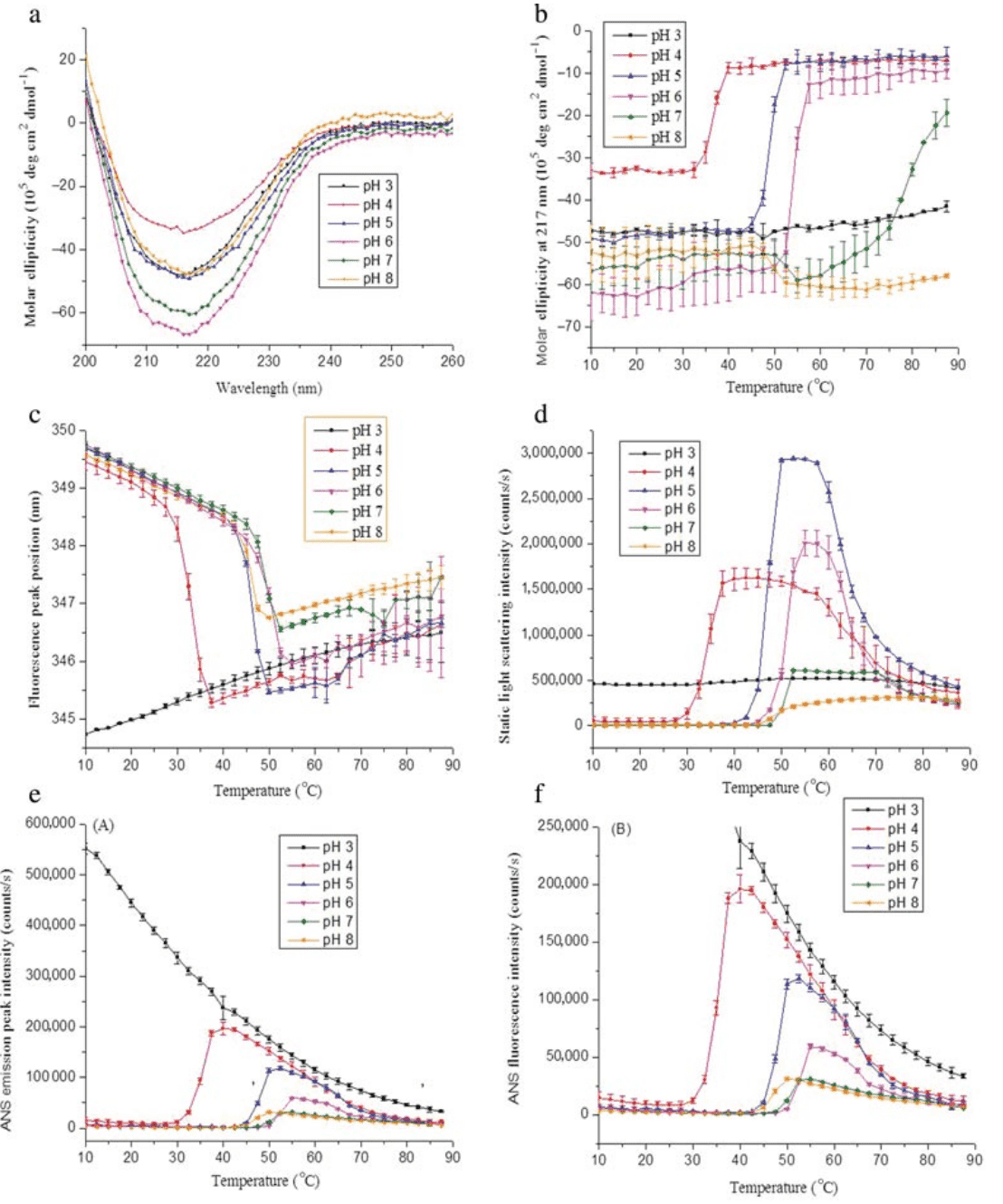
Figure 2. Biophysical Characteristics of Recombinant L460D Pneumolysoid [2]
2. Characterization of Surfactants in Oil-in-Water Emulsion-based Vaccine Adjuvants Using MS and HPLC-MS: Structural Analysis and Quantification
Mass Spectrometry (MS) and high performance liquid chromatography coupled with mass spectrometry (HPLC-MS) were developed to characterize the properties of two surfactants (cetheareth-12 and sorbitan oleate) used in the production of AF03, an emulsified oil-in-water (O/W) adjuvant. Initially, MS was used to characterize the chemical structure of these surfactants and identify their components. These surfactants appeared to be complex products, particularly regarding the nature of the fatty alcohols and the distribution of ethylene oxide units in cetheareth-12, as well as the different sorbitan-bound fatty acids (oleic, linoleic, and palmitic acids) in sorbitan oleate. Subsequently, after identifying and selecting the ions of interest, HPLC-MS was developed and optimized for quantification and "quality control" of these two surfactants, which are components of both the raw materials and the final O/W emulsion bulk and filled products.
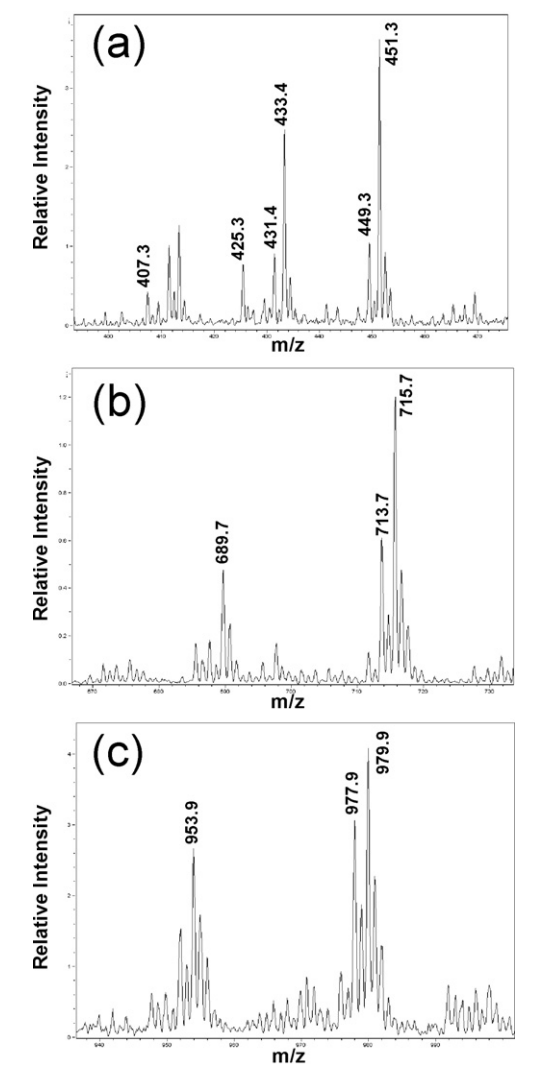
Figure 3. ESI Mass Spectrum Results of Sorbitan Oleate at 0.8 g/mL in MeOH [3]
3. Determination of Adsorbed Protein Concentration in Aluminum Hydroxide Suspensions by Near-Infrared Transmittance Spectroscopy
Analysis of aluminum hydroxide based vaccines is difficult after antigen adsorption. The adsorbed proteins are typically assessed for quality control by measuring the residual non-adsorbed proteins. This paper presents a new method using near-infrared (NIR) transmittance spectroscopy to directly measure the concentration of adsorbed proteins in suspension. A simple adsorption system using bovine serum albumin (BSA) and aluminum hydroxide was employed as the model system. The results indicated that the NIR absorbance between 700-1300 nm correlated with the concentration of adsorbed BSA measured using the UV method, and a calibration model was built using partial least squares regression (PLSR). With a 10 mm path cuvette, the linear concentration range of adsorbed BSA was from 0 to 1.75 mg/mL. This study explored the effects of suspension settling, different buffers, and different batches of aluminum hydroxide. The results showed that the main influencing factor was batch variation, while buffer variation had no impact. However, preprocessing the spectral data by subtracting the spectrum of a BSA blank control (aluminum hydroxide without BSA) significantly reduced batch effects, and NIR predictions aligned well with reference values. NIR may be the only direct method to date for determining the concentration of proteins adsorbed in suspensions. It offers a non-destructive approach, providing significant advantages for quality control and assurance in vaccine production.
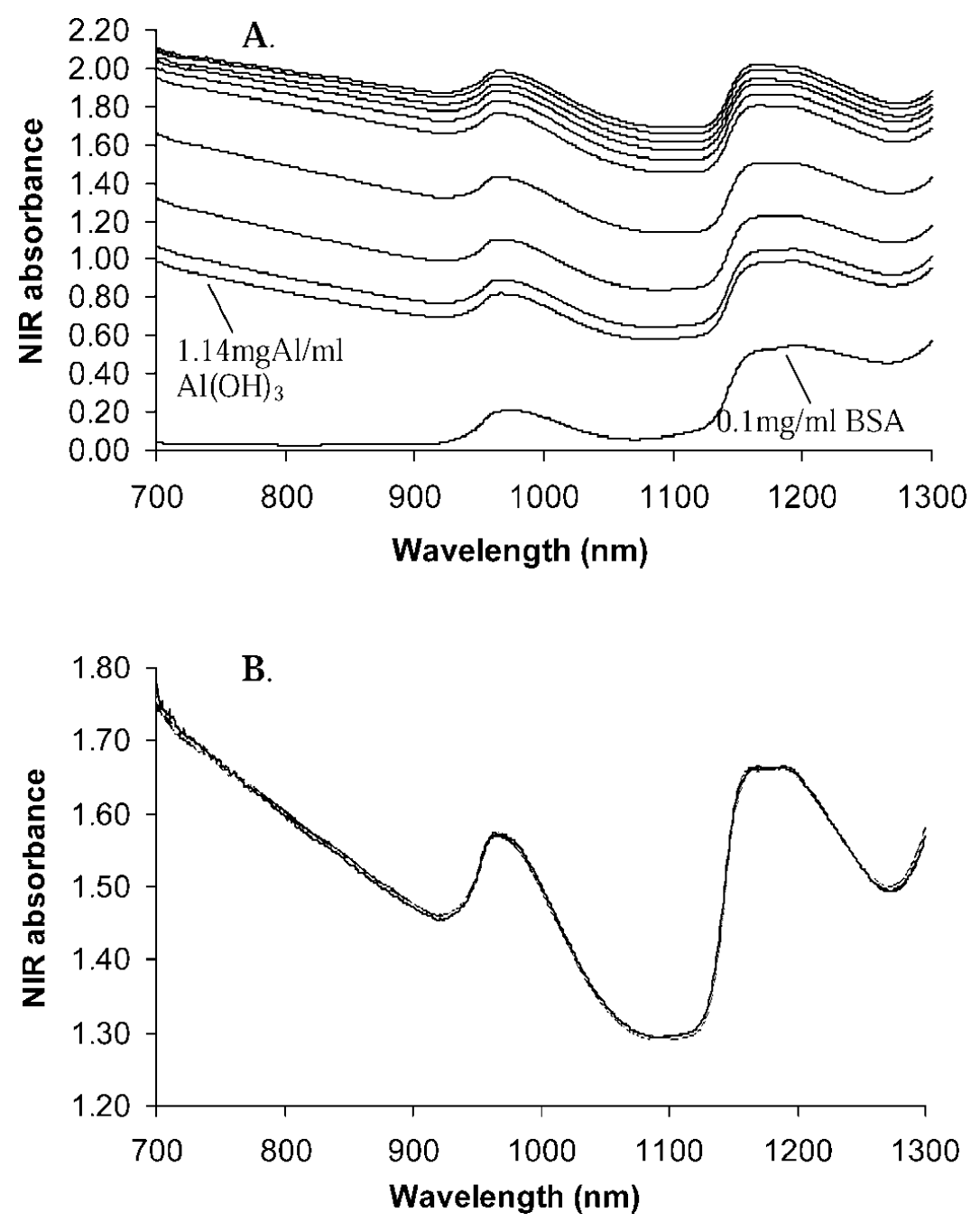
Figure 4. Typical NIR Spectral Image [4]
References
[1] Pollard AJ, Bijker EM. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol. 2021 Feb;21(2):83-100. doi: 10.1038/s41577-020-00479-7. Epub 2020 Dec 22. Erratum in: Nat Rev Immunol. 2021 Jan 5;: PMID: 33353987; PMCID: PMC7754704.
[2] Hu, Lei et al. “Physical characterization and formulation development of a recombinant pneumolysoid protein-based pneumococcal vaccine.” Journal of pharmaceutical sciences vol. 102,2 (2013): 387-400. doi:10.1002/jps.23375.
[3] Cotte, Jean-François et al. “Characterization of surfactants in an oil-in-water emulsion-based vaccine adjuvant using MS and HPLC-MS: structural analysis and quantification.” International journal of pharmaceutics vol. 436,1-2 (2012): 233-9. doi:10.1016/j.ijpharm.2012.06.018.
[4] Lai, Xuxin et al. “Determination of adsorbed protein concentration in aluminum hydroxide suspensions by near-infrared transmittance spectroscopy.” Applied spectroscopy vol. 62,7 (2008): 784-90. doi:10.1366/000370208784909481
How to order?







