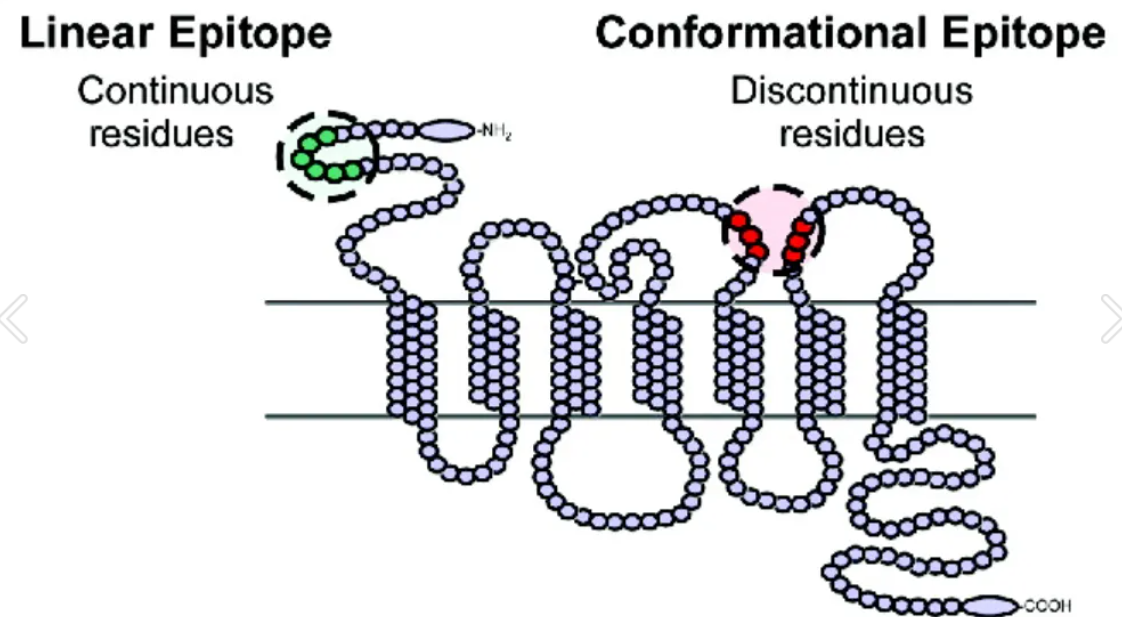
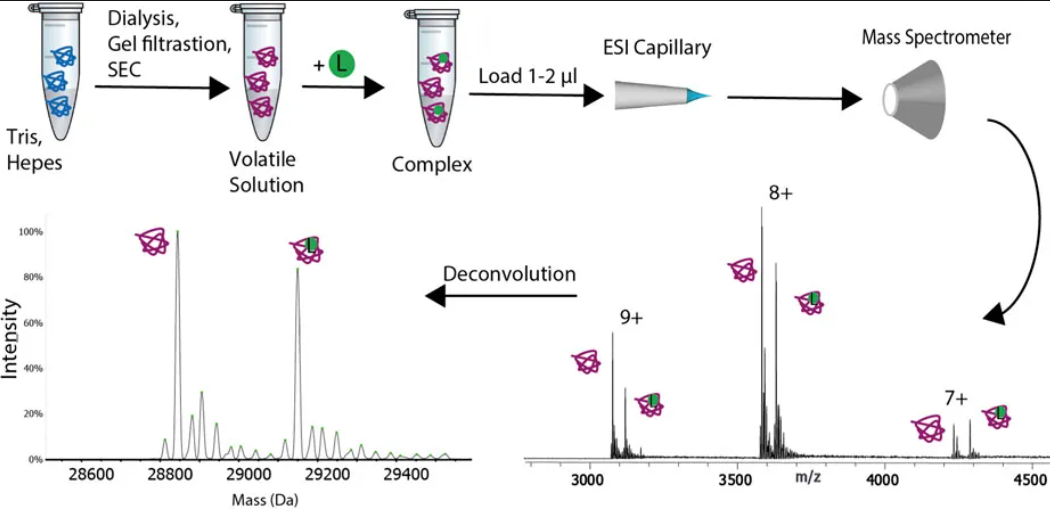
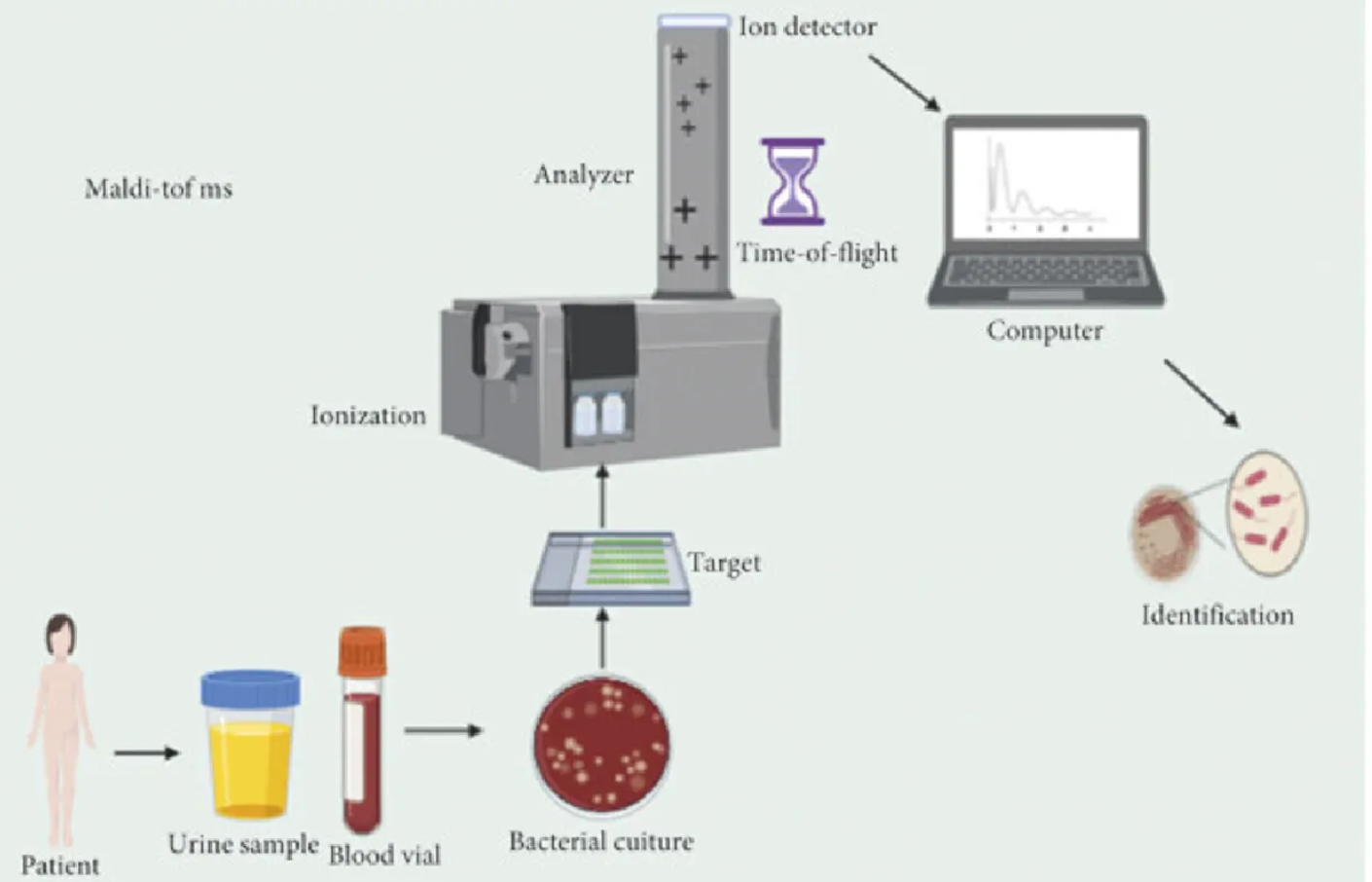
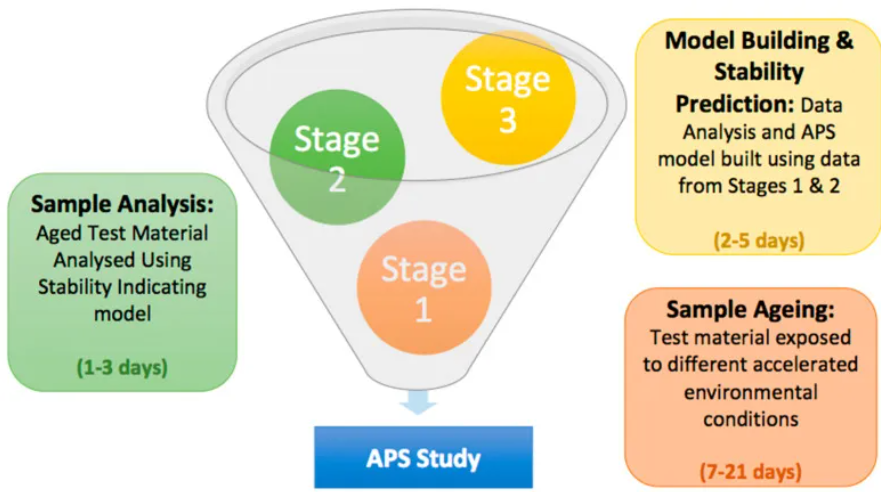
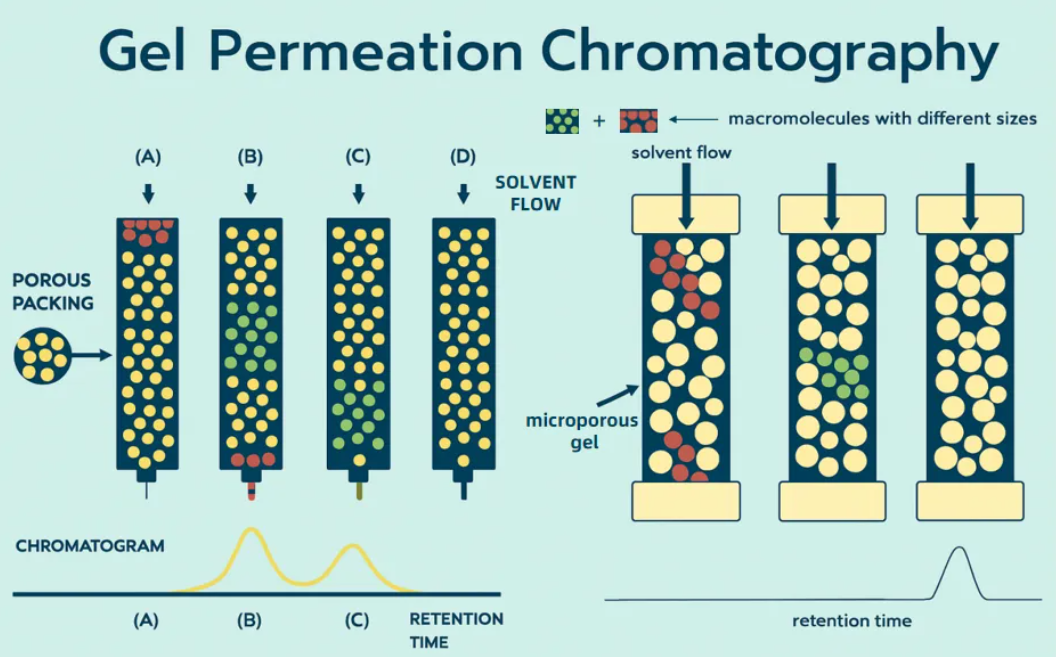
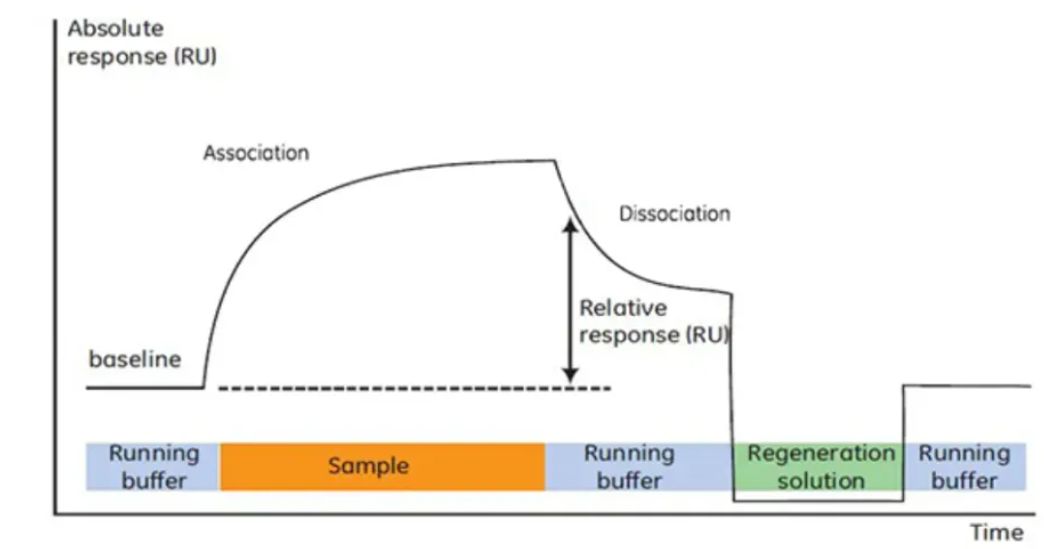
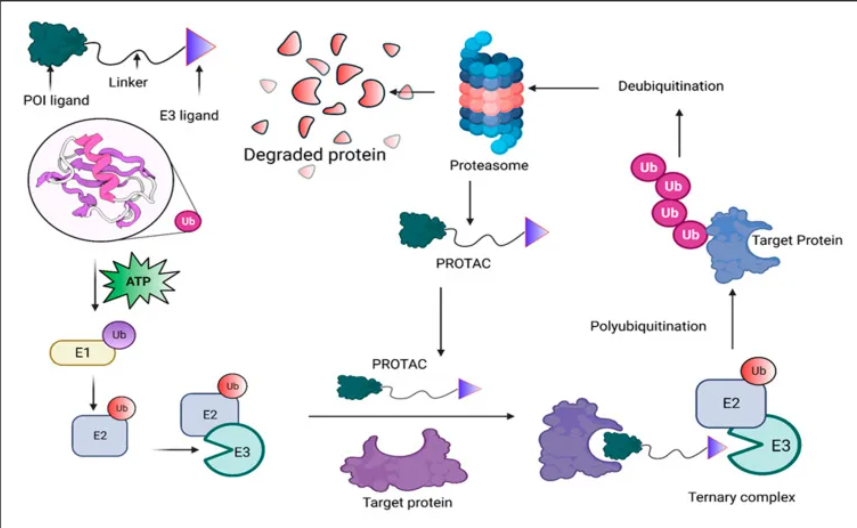
Thematic Analysis Service
Submit Inquiry
How to order?


Email:
info@MtoZ-Biolabs.comEmail:
info@MtoZ-Biolabs.com
How to order?


Submit Inquiry