Oligonucleotide Purity Analysis Services
Oligonucleotide purity analysis is a critical step for precisely determining the purity of synthesized nucleic acids such as DNA, RNA, and siRNA. Impurities can markedly compromise the quality, stability, biological activity, and experimental reproducibility of oligonucleotides. Accurate purity assessment identifies synthetic by-products, degradation fragments, and other contaminants, thus ensuring reliable downstream experiments. Given the widespread applications of oligonucleotides in gene synthesis, PCR amplification, RNA interference, gene editing, and drug development, purity assurance is essential.
MtoZ Biolabs provides precise and reliable Oligonucleotide Purity Analysis Services utilizing cutting-edge analytical platforms and an expert scientific team, empowering your research and development with rigorous quality control.
Services at MtoZ Biolabs
MtoZ Biolabs employs multiple advanced analytical techniques tailored to diverse research needs, including:
· High-Performance Liquid Chromatography (HPLC): Separates and quantifies oligonucleotides through reversed-phase, ion-exchange, or hydrophilic interaction chromatography.
· Liquid Chromatography-Mass Spectrometry (LC-MS): Provides high-resolution molecular weight analysis to characterize oligonucleotide integrity and identify impurities.
· Capillary Electrophoresis (CE): Separates oligonucleotides based on size and charge differences, ideal for rapid purity assessment.
· UV-Vis Spectroscopy: Determines total oligonucleotide concentration and evaluates potential contamination by proteins or organic solvents.
Combining these sophisticated techniques, MtoZ Biolabs ensures sensitive, precise, and reliable oligonucleotide purity analyses.
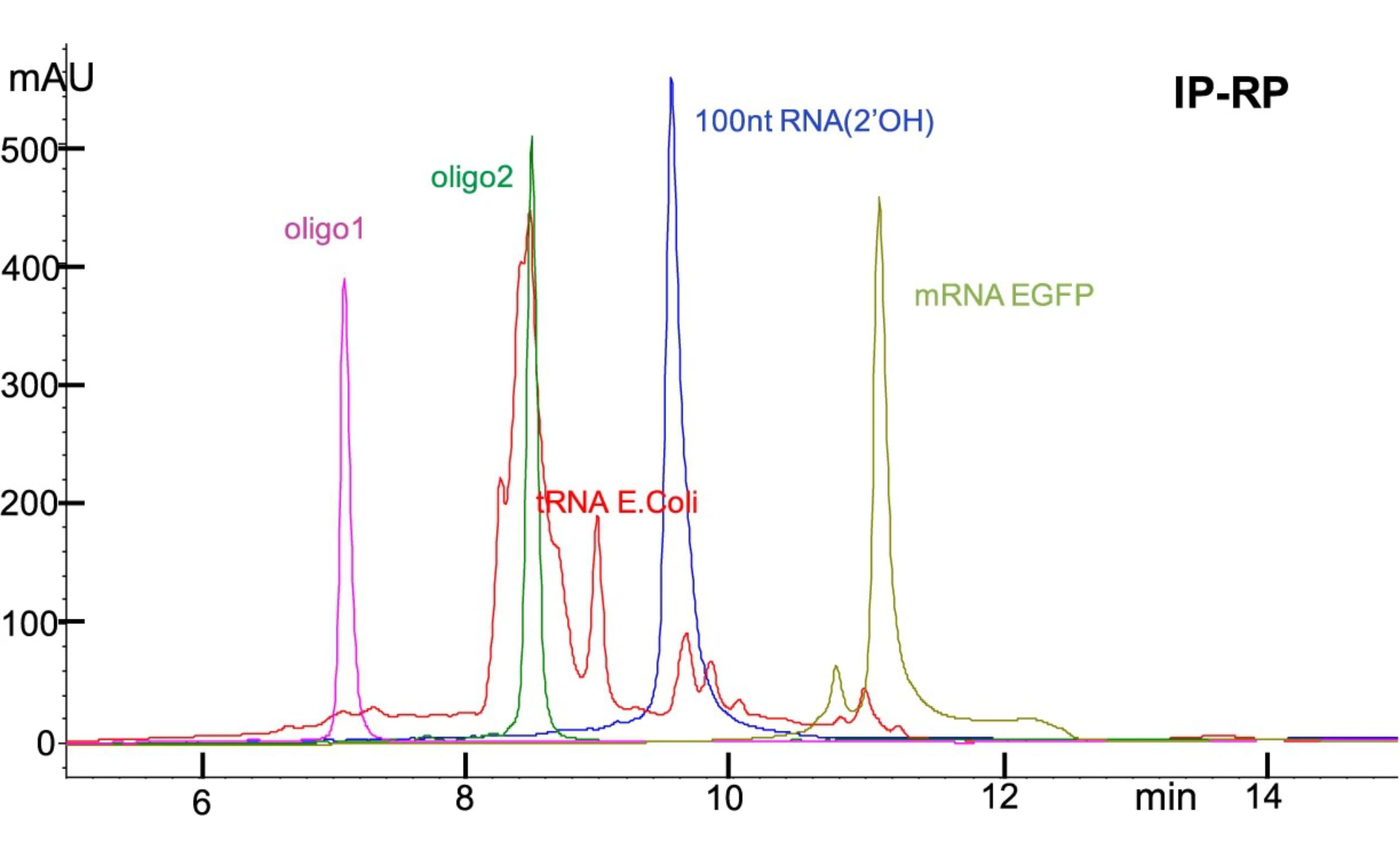
Kanavarioti, A. et al. Sci Rep. 2019.
Figure 1. HPLC-Based Purity Analysis of Oligonucleotide
Why Choose MtoZ Biolabs?
MtoZ Biolabs offers clients robust analytical expertise and advanced technical capabilities, ensuring outstanding performance in Oligonucleotide Purity Analysis Services.
1. State-of-the-Art Analytical Instruments
We utilize advanced analytical technologies, including UPLC, CE, IEC, and high-resolution mass spectrometry, ensuring high accuracy and sensitivity for research and industrial applications.
2. Customized Analytical Solutions
We tailor analytical strategies to your specific research needs, integrating multiple techniques to optimize data quality and experimental efficiency.
3. Highly Experienced Scientific Team
Our specialists bring extensive expertise in oligonucleotide analysis, ensuring rigorous experimental design, precise data interpretation, and reliable results.
4. Rapid Project Implementation
With efficient project management and seamless communication, we accelerate research timelines and streamline the path to commercialization.
5. One-Time-Charge
Our pricing is transparent, no hidden fees or additional costs.
Case Study
Case 1
This research demonstrated that two-dimensional liquid chromatography (2D-LC) coupled with mass spectrometry (MS) significantly enhances the analytical precision for antisense oligonucleotides. Researchers successfully developed a high-resolution 2D-LC/MS strategy combining ion-pair reversed-phase chromatography (IPRP), anion-exchange chromatography (AEX), and hydrophilic interaction chromatography (HILIC), enabling detection of impurities at levels as low as 0.3%. Compared to conventional IPRP-MS methods, this novel approach avoided issues associated with TEA and HFIP, improving mass spectrometry sensitivity, instrument longevity, and separation efficiency via online desalting through HILIC.
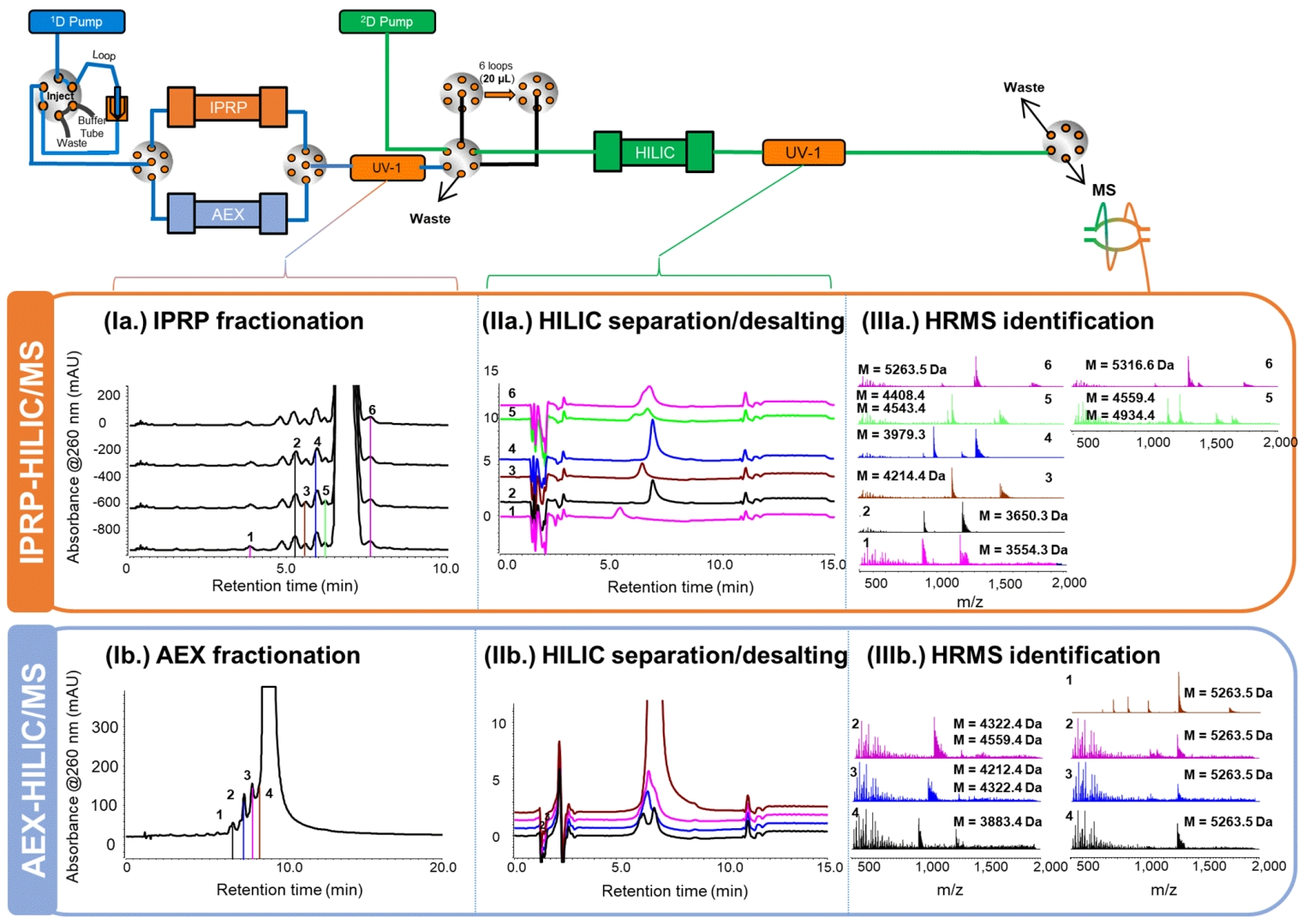
Goyon, A. et al. Anal. Chem. 2020.
This case validates the superior efficiency of the 2D-LC/MS approach, aligning seamlessly with MtoZ Biolabs' Oligonucleotide Purity Analysis Services. Leveraging advanced 2D-LC/MS platforms, we provide ultra-sensitive, high-resolution purity analyses to guarantee rigorous quality control.
As nucleic acid research continues to expand, applications for oligonucleotides have broadened significantly. MtoZ Biolabs’ Oligonucleotide Purity Analysis Services are widely utilized in nucleic acid therapeutic development, gene editing, RNA interference, molecular diagnostics, and synthetic biology research. Our rigorous purity control helps clients assure product quality at the earliest stages, facilitating successful translation of nucleic acid technologies into commercial and clinical advancements.
MtoZ Biolabs is committed to delivering precise and efficient Oligonucleotide Purity Analysis Services. For further information or customized service requests, please contact us at any time. We look forward to assisting you in achieving your research and industrial objectives.
FAQ
Q1: How do we select an appropriate oligonucleotide purity analysis method?
MtoZ Biolabs recommends selecting methods based on your specific research objectives, intended applications, and regulatory requirements. For high-sensitivity and high-resolution analyses such as drug development or quality control, chromatographic techniques like UPLC or IEC effectively distinguish target sequences from impurities. If detailed structural characterization and precise molecular weight determination are required, incorporating high-resolution mass spectrometry (e.g., ESI-MS) is strongly advised.
Q2: Can your Oligonucleotide Purity Analysis Services analyze modified oligonucleotides?
Yes. Our Oligonucleotide Purity Analysis Services fully support analysis of modified oligonucleotides, including fluorescently labeled, phosphorothioate-modified, sugar-modified, and other chemically altered nucleic acids. By combining high-performance chromatography with high-resolution mass spectrometry, we accurately distinguish modified oligonucleotide targets from impurities, ensuring rigorous quality control aligned with stringent research and regulatory standards.
Q3: What should we consider when submitting oligonucleotide samples for analysis?
Samples submitted should ideally have initial purity levels of 80% or higher, provided as dried powder or nuclease-free aqueous solutions to prevent contamination or degradation. To optimize analytical outcomes, clients should supply detailed sequence information, modification types, and potential impurity profiles. MtoZ Biolabs’s technical specialists assist with preliminary consultations and sample preparation guidance, ensuring streamlined workflows and reliable analytical results.
How to order?







