Antibody-Drug Conjugate (ADC) Sequencing Services
Antibody-drug conjugate (ADC) sequencing is a high-precision characterization technique designed to comprehensively analyze the molecular structure of ADC, including antibody amino acid sequences, conjugation sites, drug-to-antibody ratio (DAR), linker stability, and modification distribution. ADC is a class of targeted therapeutic drugs composed of monoclonal antibodies (mAbs) conjugated to cytotoxic payloads via specific linkers, enabling precise delivery of cytotoxic agents to target cells, thereby enhancing efficacy and reducing systemic toxicity. ADC sequencing analysis relies on high-resolution mass spectrometry (LC-MS/MS), intact mass analysis, peptide mapping, and glycosylation analysis to ensure accurate characterization of ADC structures and key modifications.
Antibody-drug conjugate (ADC) sequencing services are widely applied in biopharmaceutical research, antibody engineering, targeted drug development, and biosimilar studies. For instance, in ADC drug development, this analysis is used for antibody sequence identification, conjugation pattern optimization, and structural characterization. In quality control and stability studies, it helps evaluate batch consistency, degradation pathways, and conjugation homogeneity. In biosimilar development, this technique ensures structural consistency between originator and biosimilar drugs. Through high-precision sequencing, ADC analysis provides critical scientific insights for drug development, process optimization, and clinical applications, accelerating the clinical translation and commercialization of ADC therapeutics.
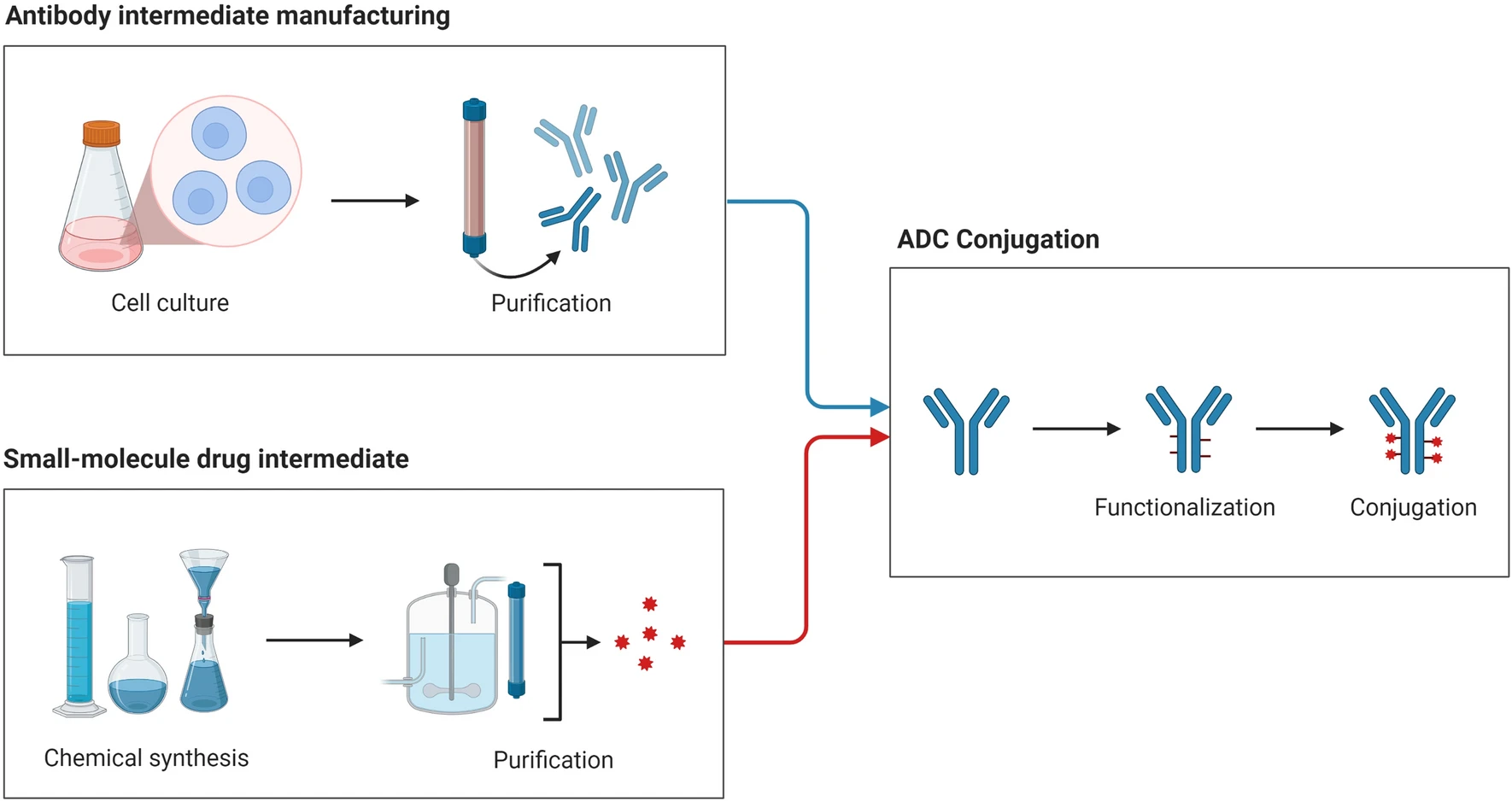
Marei, H E. et al. Cancer Cell International, 2022.
Figure 1. The Development, Purification, and Production of Antibody-Drug Conjugates.
Services at MtoZ Biolabs
Utilizing a high-resolution liquid chromatography-mass spectrometry (LC-MS/MS) platform, the antibody-drug conjugate (ADC) sequencing services provided by MtoZ Biolabs ensure high-sensitivity and high-resolution data acquisition. This service primarily covers antibody sequence analysis, drug conjugation site identification, drug-to-antibody ratio (DAR) determination, and critical quality attribute (CQA) assessment. Through optimized mass spectrometry parameters and advanced bioinformatics analysis, we deliver precise ADC structural characterization data to support the development of novel ADC therapeutics, quality control, and biosimilar research, ensuring drug consistency, safety, and efficacy.
Analysis Workflow
1. Sample Preparation
The antibody component of the ADC drug is extracted and subjected to reduction, enzymatic digestion, or deconjugation treatment to separate the antibody and drug molecules, ensuring sequencing accuracy.
2. Protein Sequencing
High-resolution liquid chromatography-mass spectrometry (LC-MS/MS) is used to precisely analyze the amino acid sequence of the antibody, post-translational modifications (PTMs), and glycosylation patterns.
3. Conjugation Site Identification
Mass spectrometry analysis is performed to detect conjugation sites on the antibody, determine the specific locations of drug attachment, and assess their distribution and drug-to-antibody ratio (DAR).
4. Data Analysis and Quantification
Bioinformatics tools are utilized to integrate antibody sequences, conjugation patterns, and modification data, assessing the structural integrity, stability, and pharmacokinetic properties of the ADC.
5. Reporting and Result Interpretation
A comprehensive ADC sequencing report is provided, including antibody sequences, conjugation sites, DAR values, and relevant modification information, supporting drug development, quality control, and biosimilar research.
Service Advantages
1. High-Resolution Precision Sequencing
Utilizing high-resolution mass spectrometry (LC-MS/MS), MtoZ Biolabs has established the antibody-drug conjugate (ADC) sequencing services platform, ensuring high-precision antibody sequence analysis and accurate identification of drug conjugation sites.
2. High Throughput
Our platform enables the simultaneous analysis of multiple samples, enhancing research efficiency and making it ideal for large-scale studies and quality control in production.
3. Comprehensive Analysis
We provide a thorough analysis of antibody sequences, drug conjugation sites, drug-to-antibody ratio (DAR), and drug components, ensuring the consistency and stability of ADC drugs.
4. Customized Analytical Solutions
Tailored experimental designs are offered based on the specific characteristics of different ADC molecules, making our services suitable for drug development, biosimilar research, and preclinical studies to meet the unique needs of our clients.
Applications
1. Drug Development and Structural Optimization
In ADC drug development, antibody-drug conjugate (ADC) sequencing services can be utilized to optimize antibody sequences, confirm conjugation sites, and determine the drug-to-antibody ratio (DAR). This enhances conjugation efficiency, refines drug design, and improves therapeutic efficacy.
2. Quality Control and Consistency Assessment
In ADC drug manufacturing and biosimilar development, sequencing can be used for batch-to-batch consistency evaluation, ensuring that ADC products meet quality standards in terms of structure, modifications, and conjugation ratios.
3. In Vivo Metabolism and Pharmacokinetics Research
Through sequencing analysis of ADC degradation patterns, conjugation stability, and drug release profiles in vivo, antibody-drug conjugate (ADC) sequencing services support pharmacokinetics (PK) and pharmacodynamics (PD) studies, aiding in the optimization of drug administration strategies.
4. Preclinical Research and Safety Assessment
In preclinical studies, sequencing can be used to evaluate ADC stability, metabolic degradation products, and potential immunogenicity, ensuring the drug's safety and efficacy.
Case Study
1. Qualitative Analysis of Antibody–Drug Conjugates (ADCs): an Experimental Comparison of Analytical Techniques of Cysteine-Linked ADCs
This study aims to experimentally compare analytical methods for cysteine-linked antibody-drug conjugate (ADC) to evaluate the applicability and advantages of different techniques in ADC characterization. The research focuses on various cysteine-linked ADC molecules with different drug-to-antibody ratios (DAR) and structural features. The study employs multiple analytical techniques, including high-performance liquid chromatography (HPLC), liquid chromatography-mass spectrometry (LC-MS), capillary electrophoresis (CE), and size-exclusion chromatography (SEC), to systematically assess ADC purity, homogeneity, DAR distribution, and protein structural integrity. The results indicate that each technique has specific advantages in detecting different ADC attributes: LC-MS provides precise determination of DAR and molecular weight distribution, HPLC is suitable for overall characterization and stability analysis, CE effectively analyzes charge variants, and SEC evaluates aggregate formation. The study concludes that different analytical techniques have unique strengths in ADC quality control and structural characterization, and a combination of multiple methods offers a more comprehensive and accurate assessment, providing essential technical support for ADC development and quality control.
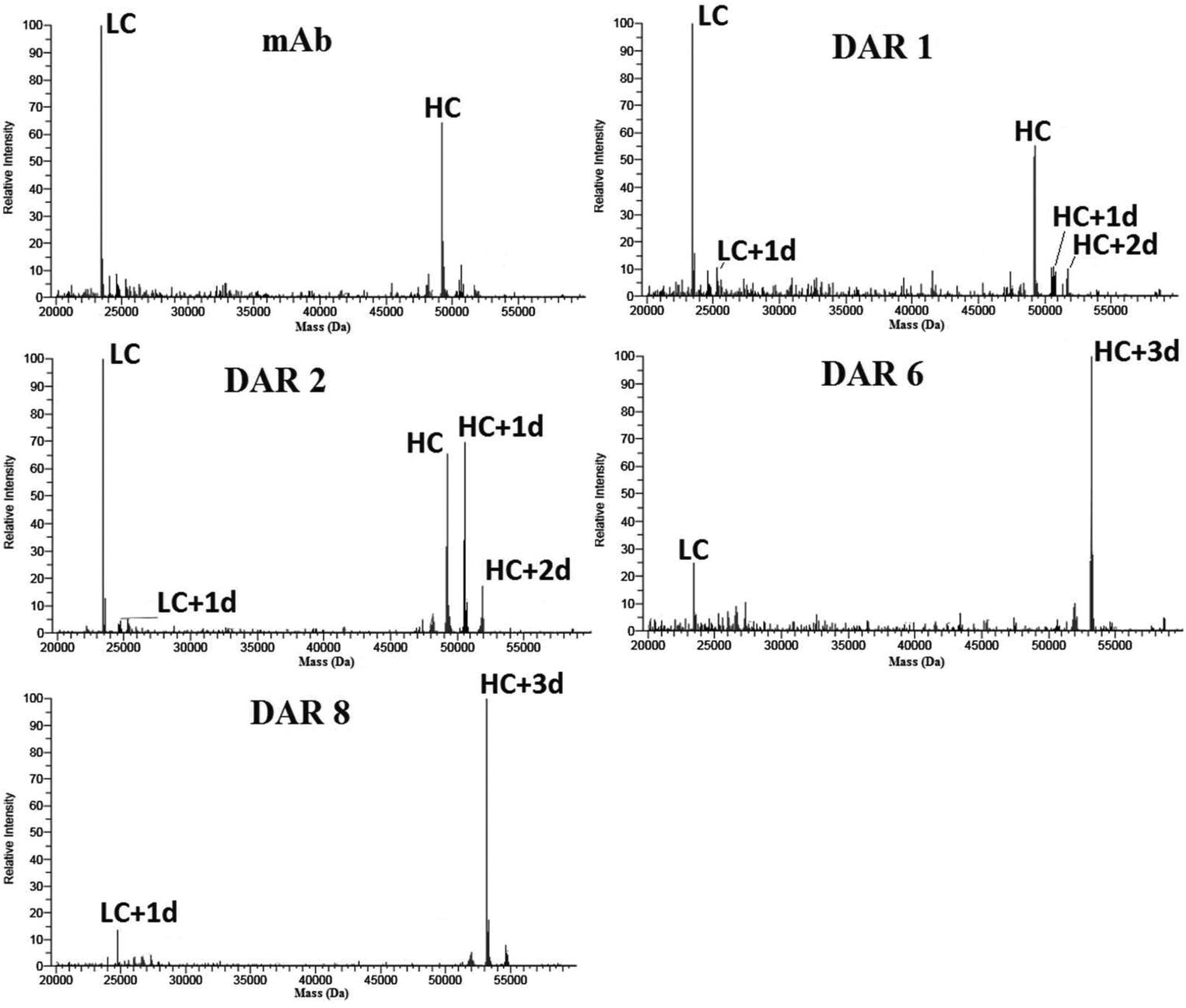
Källsten, M. et al. Analyst, 2018.
Figure 2. Deconvolution Spectra of Light and Heavy Chains of Unbound Antibodies and ADC by RPLC-MS (Orbitrap).
Deliverables
1. Experimental Procedures
2. Relevant Mass Spectrometry Parameters
3. Detailed Information on Antibody-Drug Conjugate (ADC) Sequencing
4. Mass Spectrometry Images
5. Raw Data
FAQ
Q1: Can ADC sequencing identify all types of drug conjugation sites?
A1: Different types of ADCs (such as lysine-conjugated, cysteine-conjugated, and non-natural amino acid-conjugated ADCs) require specific enzymatic digestion strategies and data analysis methods to achieve optimal sequencing results.
Q2: How does ADC sequencing differ from traditional antibody sequencing?
A2: ADC sequencing not only involves determining the full sequence of the antibody but also analyzes the conjugated drug molecules and their linkage sites. In contrast, traditional antibody sequencing primarily focuses on identifying the amino acid sequences of the antibody’s light and heavy chains, without addressing drug conjugation.
MtoZ Biolabs, an integrated Chromatography and Mass Spectrometry (MS) Services Provider, provides advanced proteomics, metabolomics, and biopharmaceutical analysis services to researchers in biochemistry, biotechnology, and biopharmaceutical fields. Our ultimate aim is to provide more rapid, high-throughput, and cost-effective analysis, with exceptional data quality and minimal sample consumption. Free project evaluation, welcome to learn more details!
How to order?







