AAV Characterization Service
- Capsid Protein Identification and Quantification: Detailed analysis of VP1, VP2, and VP3 proteins, including their ratios and post-translational modifications.
- Genome Integrity Assessment: Verification of the vector genome sequence and confirmation of genetic fidelity using advanced sequencing technologies.
- Purity and Impurity Profiling: Detection and quantification of host cell proteins, nucleic acid contaminants, and empty or partially filled capsids.
- Stability Studies: Evaluation of vector stability under various conditions to support formulation development and shelf-life determination.
- Aggregation Analysis: Aggregation can impact the efficacy and safety of AAV vectors. We analyze particle size distribution and detect aggregates to ensure product consistency.
- Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS)
- High-Performance Liquid Chromatography (HPLC)
- Size-Exclusion Chromatography (SEC)
- Ion Exchange Chromatography (IEX)
- Analytical Ultracentrifugation (AUC)
- Enzyme-Linked Immunosorbent Assay (ELISA)
- Dynamic Light Scattering (DLS)
- Surface Plasmon Resonance (SPR)
- Capillary Electrophoresis (CE)
- UV-Visible Spectroscopy (UV-Vis)
Comprehensive AAV Characterization is crucial to meet stringent regulatory requirements and ensure the safety, purity, and efficacy of gene therapy products. Regulatory bodies such as the U.S. Food and Drug Administration and the European Medicines Agency (EMA) mandate thorough documentation of vector composition, potency, purity, and stability before approving AAV-based therapeutics. Given the complex structure of AAVs—with capsids composed of three viral proteins (VP1, VP2, and VP3) in specific ratios—variations in capsid assembly and genome packaging can lead to heterogeneity in the final product. Post-translational modifications and the presence of empty or partially filled capsids add to this complexity, making accurate AAV characterization both challenging and essential. Meticulous quality control through robust characterization methods ensures batch-to-batch consistency, which is vital for reproducibility and reliability in therapeutic applications. Detecting and quantifying impurities such as residual host cell proteins, DNA contaminants, and empty capsids helps to prevent adverse immune responses and maintain the integrity of the gene therapy product, thereby ensuring patient safety.
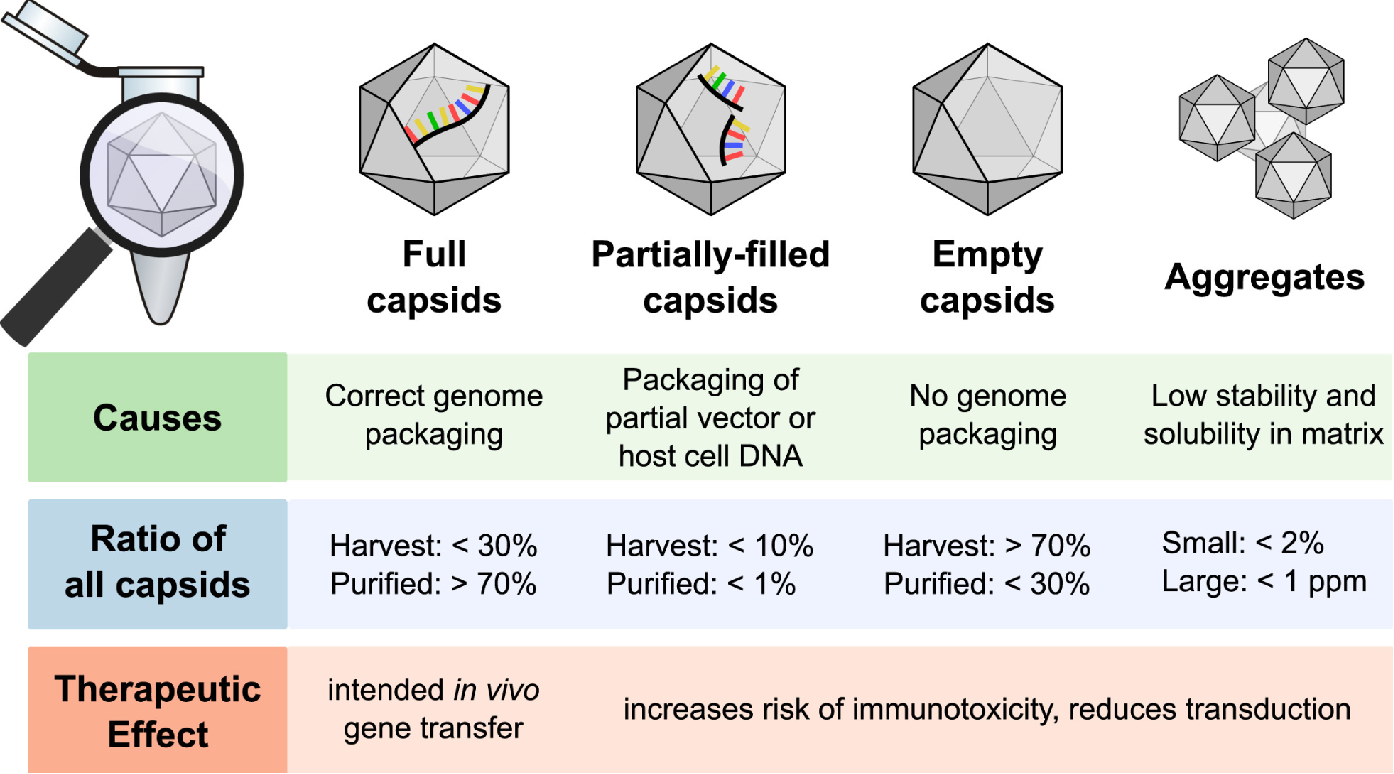
Gimpel, A. L. et al. Mol Ther Methods Clin Dev. 2021.
Figure 1. Overview of the Main Types of Capsids Generated during rAAV Production
Discovered in the 1960s as dependoparvoviruses requiring a helper virus to replicate, AAVs are non-pathogenic and exhibit low immunogenicity in humans. Their ability to infect both dividing and non-dividing cells enables efficient transduction across a wide range of tissues. With multiple serotypes and engineered variants available, AAVs can be tailored for targeted delivery to specific cell types or organs, enhancing therapeutic efficacy while minimizing off-target effects. This specificity and adaptability make AAVs the preferred vectors in clinical applications for genetic disorders, neurological diseases, and ocular conditions. AAV Characterization Service helps us understand their properties and optimize their use as viral vectors.
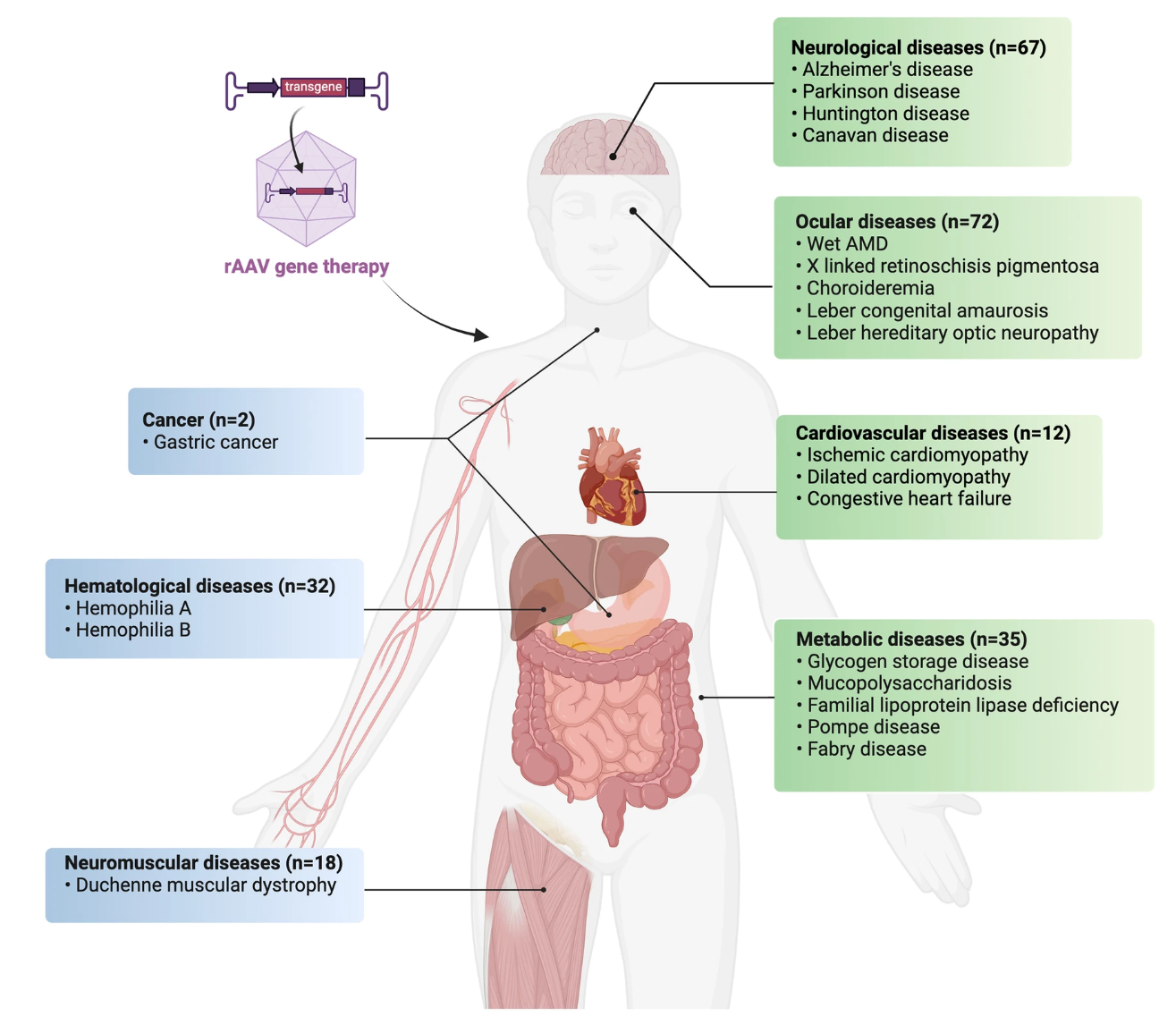
Figure 2. Current Clinical Applications of rAAV in Major Human Diseases
Service at MtoZ Biolabs
As gene therapy continues to advance, the precise characterization of Adeno-Associated Virus (AAV) vectors becomes increasingly crucial. To address this essential need, MtoZ Biolabs provides comprehensive AAV Characterization Service to support your gene therapy development, regulatory compliance, and quality assurance initiatives. Our AAV Characterization Service includes detailed identification and quantification of capsid proteins, purity and impurity analysis, stability studies, and aggregation assessment. We utilize techniques such as intact mass analysis, peptide mapping, and nucleic acid sequencing to deliver a complete profile of your AAV products.
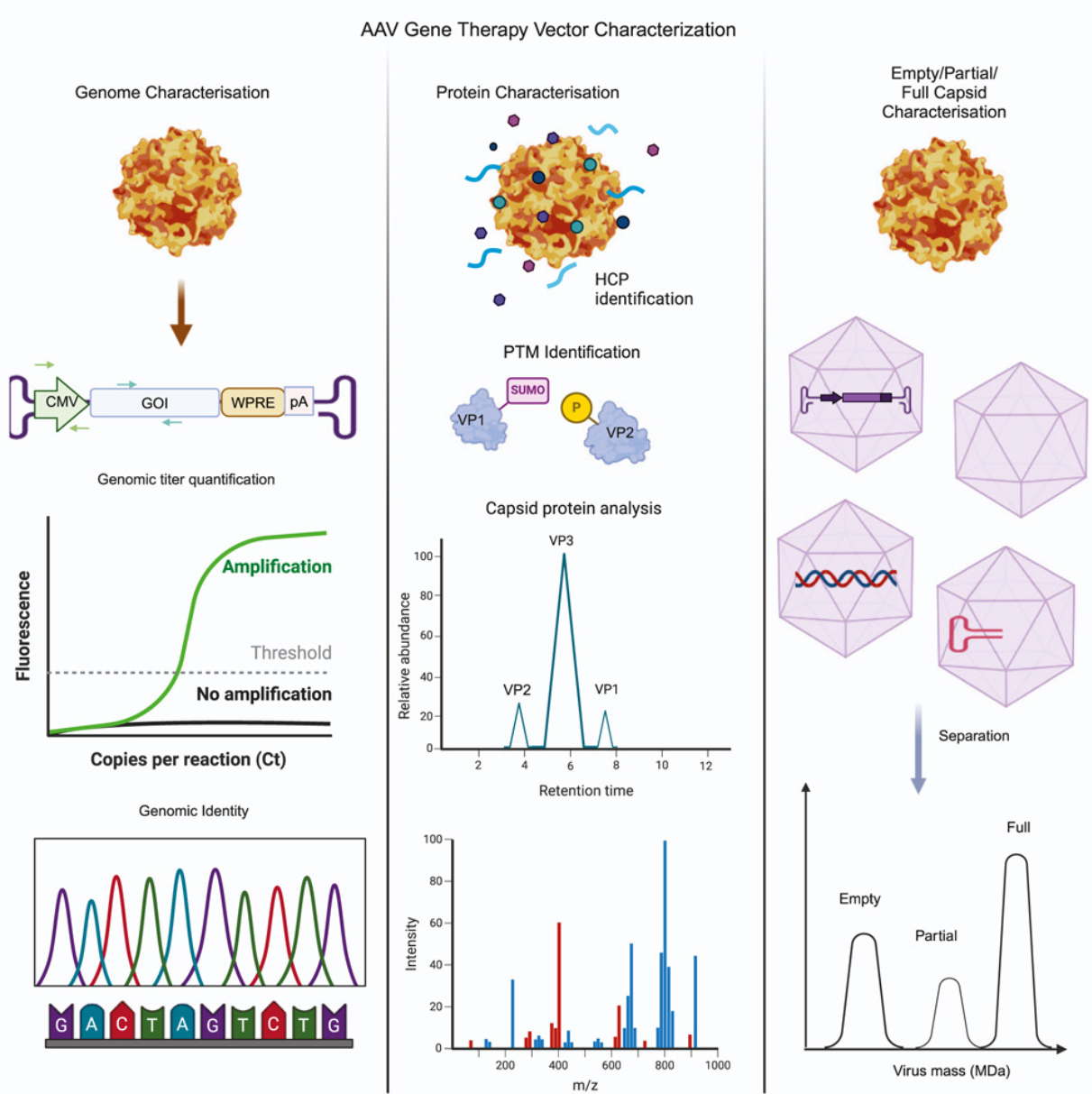
Kontogiannis, T. et al. Mol Ther Methods Clin Dev. 2024.
Figure 3. Analytical Techniques and Critical Quality Attributes of AAV Characterization
Advanced Analytical Platforms at MtoZ Biolabs
Service Advantages
1. Advance Analysis Platform: MtoZ Biolabs established an advanced AAV Characterization Service platform, guaranteeing reliable, fast, and highly accurate analysis service.
2. One-Time-Charge: Our pricing is transparent, no hidden fees or additional costs.
3. High-Data-Quality: Deep data coverage with strict data quality control. AI-powered bioinformatics platform integrates all AAV characterization data providing clients with a comprehensive data report.
4. Customized Reporting: Provides detailed, client-specific reports to support informed research decisions.
5. Rapid Turnaround: Efficient workflows ensure quick and accurate delivery of results.
Applications
Our AAV Characterization Service supports a wide range of critical applications:
1. Gene Therapy Development: Optimize vector design and gene delivery efficiency.
2. Preclinical Research: Assess safety and efficacy in animal models.
3. Clinical Trial Support: Ensure regulatory compliance and batch consistency.
4. Vector Optimization: Engineer capsids for better targeting and reduced immunogenicity.
5. Biotechnology Development: Assist in developing AAV-based therapeutics.
Case Study
1. Precise Capsid Quantification with AUC
Analytical Ultracentrifugation (AUC) was utilized to analyze recombinant AAV (rAAV) samples before and after preparative ultracentrifugation. The initial sample (OXBS1 AEX product) showed 16.5% empty capsids, 26.1% intermediate capsids, and 57.4% full capsids, highlighting the need for optimization. After ultracentrifugation, the OXBS1-F pool achieved 91.1% full capsids with minimal impurities (0.3% empty and 8.6% intermediate), indicating successful enrichment. The OXBS1-I pool contained 55.8% intermediate capsids, while the OXBS1-E pool consisted of 83.0% empty capsids, showcasing effective separation. Using the Optima AUC system, sedimentation coefficients (S) were quantified, allowing precise identification and relative quantification of each capsid type. This study underscores the precision of our AAV Characterization Service in optimizing AAV quality attributes, such as full capsid enrichment, to ensure high-quality products for superior therapeutic outcomes.
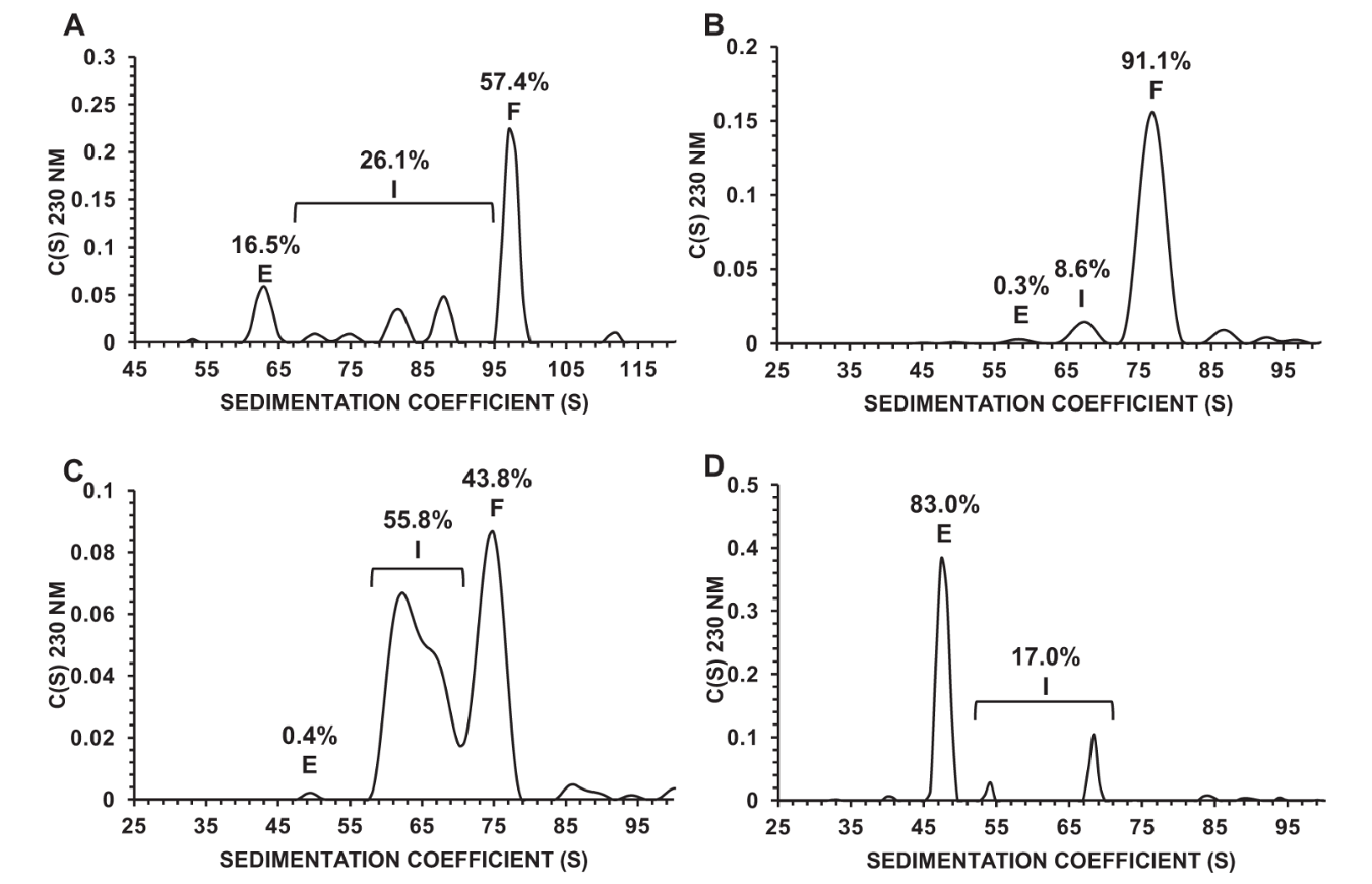
McColl-Carboni, A. et al. Gene Ther. 2024.
Figure 4. Capsid Content Determination by AUC
2. AAV Capsid Differentiation Using SEC-UV
In this study, size-exclusion chromatography (SEC) combined with UV detection is employed to differentiate heavy and light AAV capsids based on their A260/A280 ratios. SEC effectively separated AAV capsids by size, with monomeric capsids eluting at ~10.5 minutes, higher-order multimers and impurities eluting earlier (<10 minutes), and buffer components and small nucleotide impurities eluting later (>12 minutes). For heavy capsid samples, the A260/A280 ratio was ~1.34, indicating the presence of fully packaged capsids with a high DNA-to-protein ratio. In contrast, light capsid samples exhibited similar elution profiles but showed a significantly reduced A260 absorbance, resulting in an A260/A280 ratio of ~0.6. This lower ratio is consistent with capsids containing minimal or no DNA. This study demonstrates the effectiveness of SEC-UV analysis in distinguishing heavy and light capsids based on their composition. By combining A260/A280 ratio measurements with precise separation of monomers and dimers, MtoZ Biolabs provides reliable AAV Characterization Service, enabling accurate quality control and optimization for AAV-based gene therapy vectors.
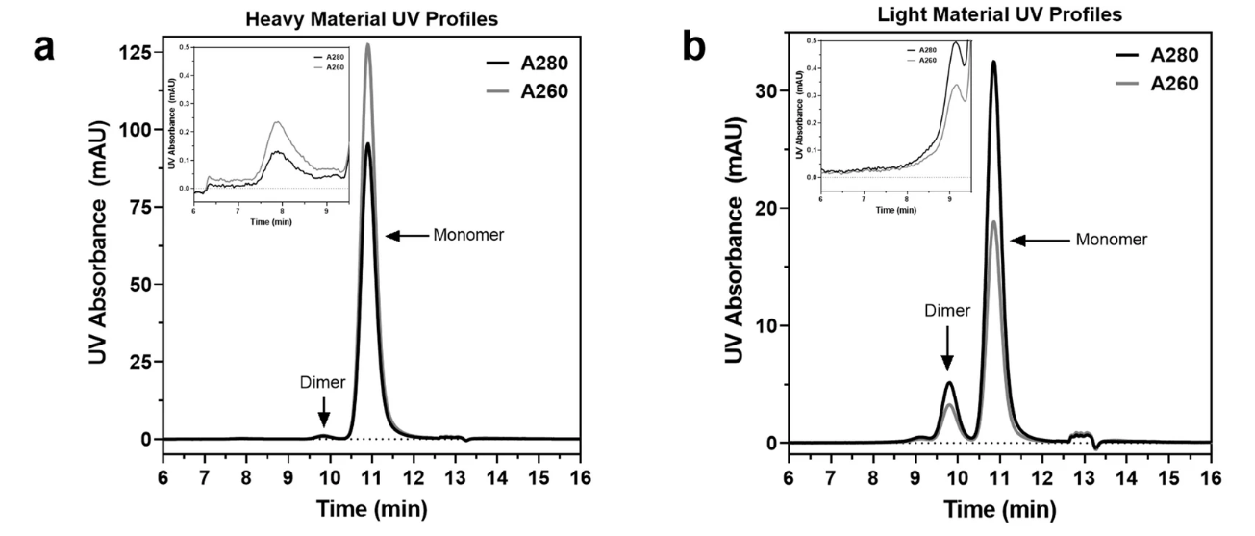
McIntosh, N. L. et al. Sci Rep. 2021.
Figure 5. 280 nm (–) and 260 nm (–) Absorbance Profiles of a (a) Heavy and (b) Light AAV Sample
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Relevant Liquid Chromatography and Mass Spectrometry Parameters
4. The Detailed Information of AAV Characterization
5. Mass Spectrometry Image
6. Raw Data
At MtoZ Biolabs, we are committed to providing high-quality, reliable, and personalized analysis services. If you are interested in our AAV Characterization Service, please feel free to contact us.
How to order?







