Chemical Industry
The chemical industry serves as the foundation for numerous global sectors, from pharmaceuticals, materials science, and energy production to agriculture and consumer goods. It drives innovation, sustainability, and industrial development by transforming raw substances into valuable products and intermediates. As chemical manufacturing grows more advanced and regulations become more stringent, reliable analytical testing has become indispensable to ensuring product quality, safety, compliance, and performance.
MtoZ Biolabs provides a full spectrum of analytical and testing solutions tailored to the needs of the chemical industry. Combining mass spectrometry, chromatography, spectroscopy, and thermal analysis platforms, we deliver precise and reproducible data for a broad range of materials, including fine chemicals, industrial reagents, catalysts, polymers, solvents, additives, and environmental samples. Our integrated analytical platform supports clients throughout every stage of product development, quality control, and regulatory assessment, helping transform scientific concepts into market-ready innovations.
Service at MtoZ Biolabs
MtoZ Biolabs, with its advanced analytical infrastructure and multidisciplinary expertise, bridges the gap between laboratory analysis and industrial application. Our services empower chemical manufacturers, researchers, and regulatory professionals to make data-driven decisions that enhance product integrity, accelerate development timelines, and minimize risk. MtoZ Biolabs integrates high-performance analytical technologies with expert consultation to provide end-to-end testing solutions for the chemical industry. Our services cover the entire product lifecycle, from raw material verification to long-term stability evaluation.
1. Compositional and Purity Analysis
Analytical Methods:
🔸Chromatographic separation: High-performance liquid chromatography (HPLC), gas chromatography (GC), and ion chromatography (IC) for quantitative purity assessment and impurity profiling.
🔸Spectroscopic identification: Nuclear magnetic resonance (NMR) and Fourier-transform infrared (FTIR) spectroscopy for molecular structure elucidation.
🔸Mass spectrometric confirmation: LC-MS/MS and GC-MS for molecular weight determination and trace-level impurity detection.
Applications:
Authentication of raw materials and intermediates.
Verification of product batch consistency.
Quantification of residual solvents and impurities in fine and specialty chemicals.
Figure 1. Purity Analysis of Biopharmaceuticals by SEC
2. Structural and Molecular Characterization
Analytical Methods:
🔸Spectroscopic techniques: FTIR, Raman, UV-Vis, and NMR to identify functional groups, molecular bonds, and structural conformation.
🔸Mass spectrometry: High-resolution LC-MS/MS for detailed molecular fragmentation analysis.
🔸X-ray diffraction (XRD): Determination of crystalline phase and molecular lattice parameters.
Applications:
Structure confirmation of synthesized compounds.
Comparative evaluation of polymorphic or isomeric forms.
Product identification in polymer, resin, and catalyst research.
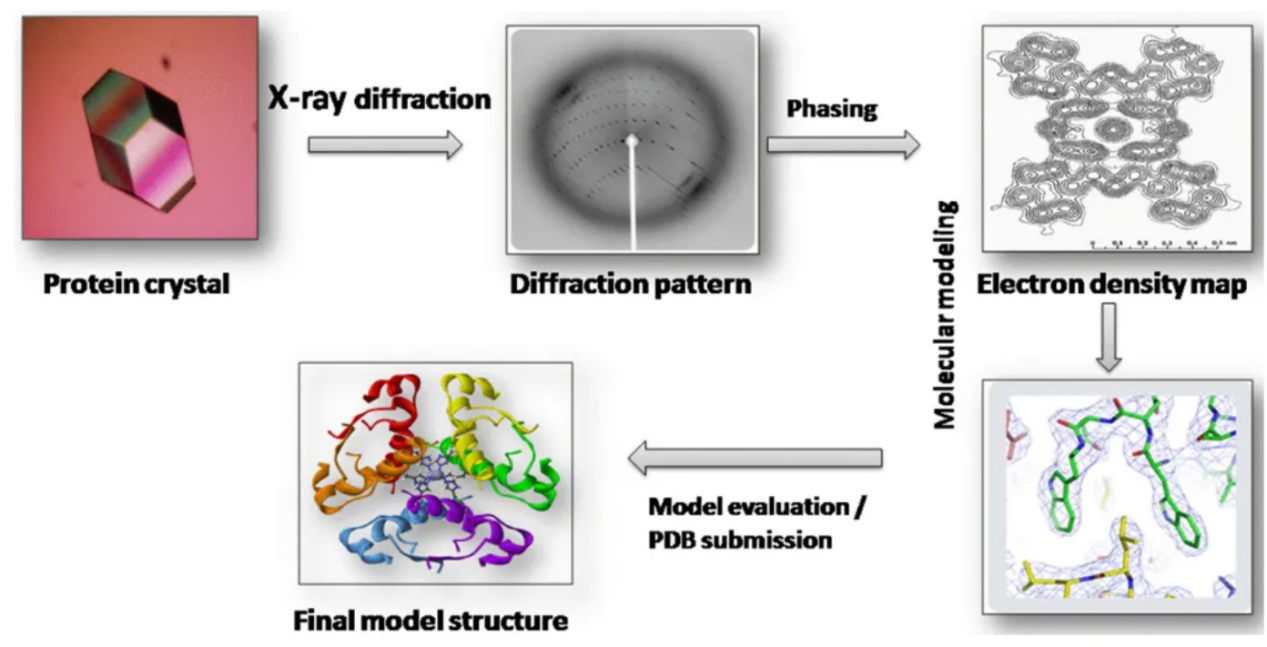
Umesh, B. et al. 2019.
Figure 2. Workflow of X-Ray Crystallography Protein Structure Determination
3. Stability Evaluation
Analytical Methods:
🔸Thermal Stability Assessment: Use differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), and differential thermal analysis (DTA) to evaluate melting points, phase transitions, and decomposition temperatures of chemical substances.
🔸Chemical Stability Assessment: Use LC-MS/MS, HPLC, and UV-Vis spectroscopy to monitor degradation kinetics and identify degradation products under stress conditions.
Applications:
Shelf-life and storage stability assessment of industrial chemicals.
Quality assurance for pharmaceutical raw materials and intermediates.
Preservation studies for food additives and packaging materials.
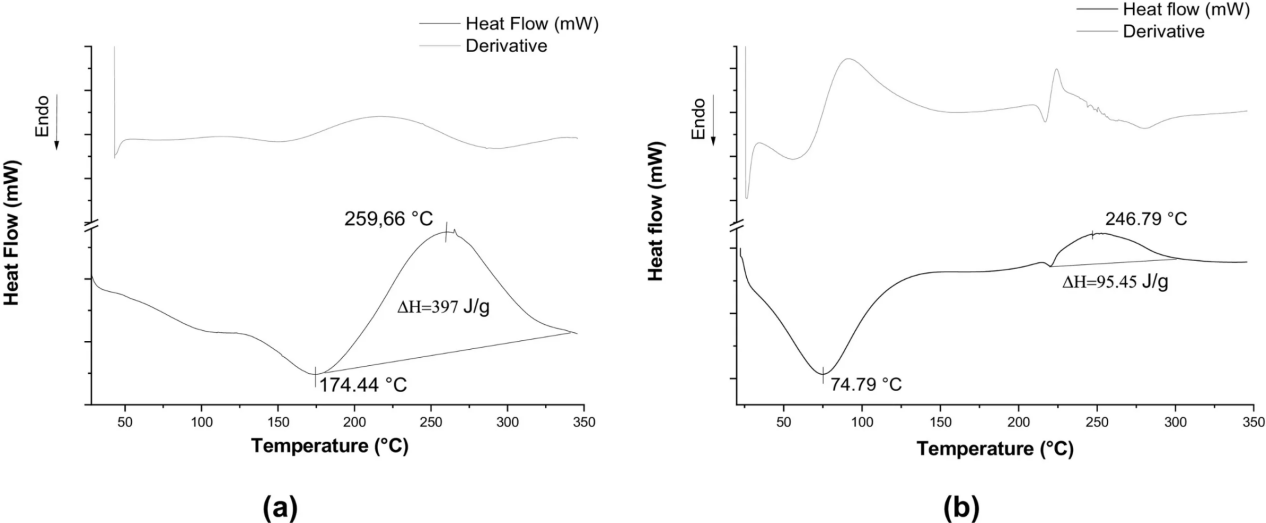
Cerón, A. A. et al. Int J Biol Macromol. 2021.
Figure 3. Differential Scanning Calorimetry (DSC) Microspheres (a) Chitosan Microspheres (CMS) and (b) Chitosan-Lysozyme Microspheres (C-LMS)
4. Material Property and Performance Testing
Analytical Methods:
🔸Rheology and viscosity measurements for evaluating flow properties and polymer behavior.
🔸Mechanical and surface characterization using scanning electron microscopy (SEM), atomic force microscopy (AFM), and nanoindentation testing.
🔸Thermal-mechanical analysis combining TGA/DSC with dynamic mechanical analysis (DMA) for mechanical performance under stress.
Applications:
Product optimization in polymer, coating, and adhesive industries.
Correlation of structural properties with mechanical strength and elasticity.
Development of new high-performance materials and composites.
5. Contaminant and Residual Analysis
Analytical Methods:
🔸ICP-MS and ICP-OES for trace metal detection and elemental composition.
🔸GC-MS and LC-MS/MS for residual solvent, pesticide, and volatile impurity screening.
🔸Ion chromatography (IC) for ionic contaminants and inorganic residues.
Applications:
Detection of heavy metals in industrial chemicals and environmental samples.
Validation of purity in catalyst formulations and reaction intermediates.
Monitoring process cleanliness and environmental impact in chemical manufacturing.
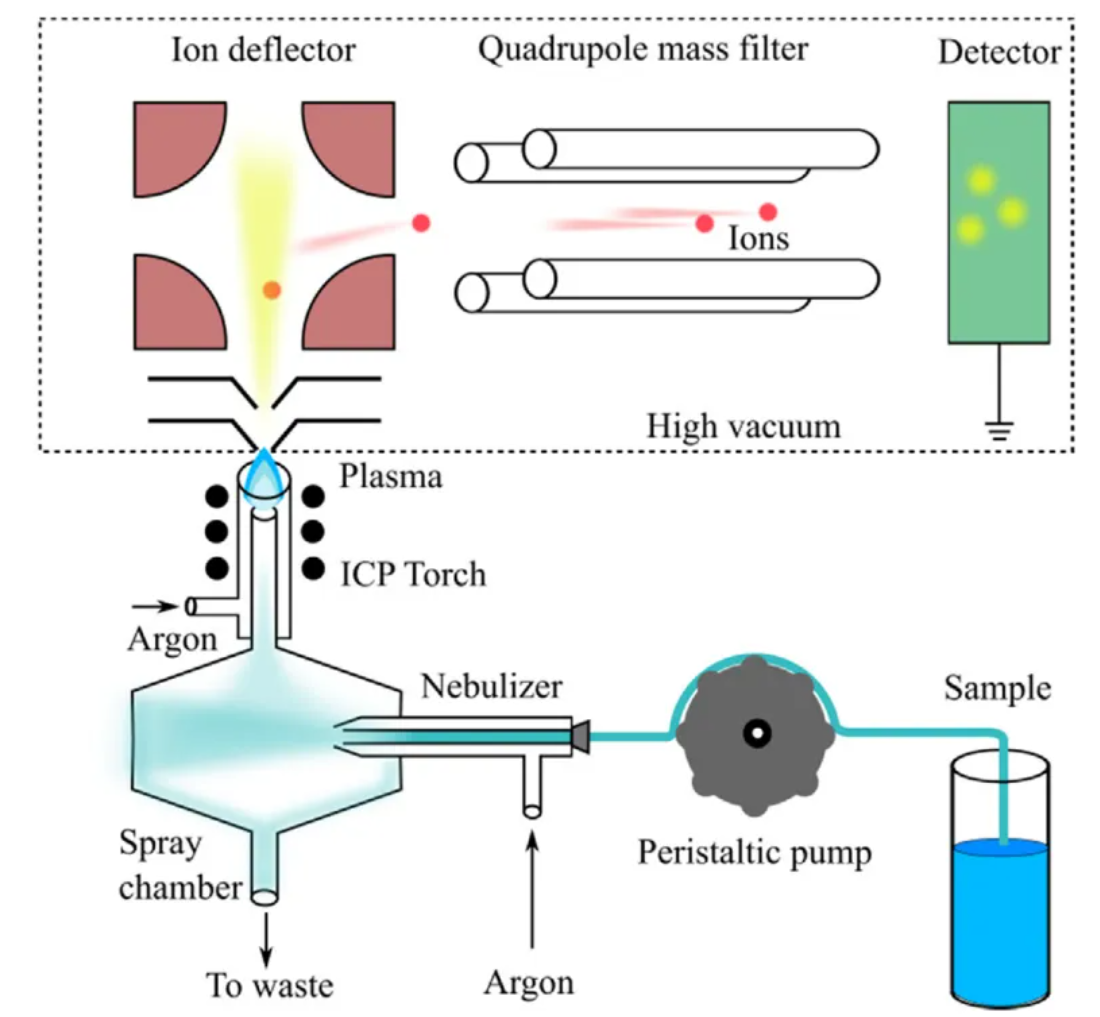
Cherevko, S. et al. Curr. Opin. Electrochem. 2023.
Figure 4. A Simplified Scheme Showing the Basic Operational Principles of ICP-MS
6. Reaction and Process Monitoring
Analytical Methods:
🔸In-situ FTIR and UV-Vis spectroscopy to monitor real-time chemical reaction kinetics.
🔸Online GC and LC analysis for intermediate and product quantification.
🔸Mass spectrometry to track molecular transformation and reaction pathways.
Applications:
Optimization of synthesis efficiency and yield.
Process troubleshooting during scale-up or batch production.
Kinetic modeling for industrial chemical manufacturing.
7. Degradation Pathway and Product Impurity Profiling
Analytical Methods:
🔸LC-MS/MS coupled with chromatographic fractionation for degradation product identification.
🔸NMR spectroscopy for elucidating structural changes in degraded compounds.
🔸UV-Vis and FTIR for detecting oxidative and photolytic by-products.
Applications:
Evaluation of degradation mechanisms under thermal, oxidative, or photochemical stress.
Identification of potential hazardous by-products in industrial formulations.
Regulatory documentation for chemical product safety and environmental compliance.
Service Advantages
✔️Customizable Analytical Solutions
Tailored project design based on sample type, matrix complexity, and client-specific requirements.
✔️High Sensitivity and Accuracy
State-of-the-art instrumentation delivers precise and reproducible results, even for trace impurities or complex mixtures.
✔️End-to-End Project Support
From method development and validation to data interpretation and compliance documentation, our team ensures seamless project execution.
✔️Rapid Turnaround and Transparent Communication
Efficient workflows and real-time client updates ensure timely delivery and full visibility throughout every analytical phase.
Sample Submission Suggestions
1. Sample Types: Liquids, solids, powders, polymers, catalysts, and environmental materials.
2. Sample Quantity: Typically 1–5 grams or milliliters per sample, depending on the analysis method.
MtoZ Biolabs also provides detailed sample submission instructions and customized handling protocols for sensitive or reactive materials. For other special sample types or low-yield materials, please contact us before preparation.
FAQ
Q1: How are thermally unstable or volatile compounds analyzed without degradation?
For heat-sensitive or volatile analytes, MtoZ Biolabs employs cryogenic sample handling, headspace GC-MS, and low-temperature injection systems to minimize decomposition. Derivatization techniques or solid-phase microextraction (SPME) can also be applied to stabilize analytes and enhance recovery while maintaining molecular integrity.
Q2: How do you evaluate the compatibility of chemical products with storage containers or packaging materials?
We conduct extractables and leachables studies using GC-MS, LC-MS/MS, and ICP-MS to identify potential compounds migrating from packaging into chemical products. These analyses are critical for ensuring long-term product integrity, particularly for reactive chemicals, solvents, and high-purity materials.
Q3: Can MtoZ Biolabs analyze reactive intermediates or transient species during synthesis?
Yes. Time-resolved and in-situ analytical methods, including rapid-scan FTIR and online LC-MS, allow detection of short-lived intermediates during reaction progress. These data provide insight into reaction mechanisms and kinetic bottlenecks, supporting process optimization in industrial chemistry.
Q4: How are low-concentration impurities differentiated from background noise?
Advanced data deconvolution algorithms combined with high-resolution mass spectrometry enable accurate distinction between true analyte signals and background interference. The use of isotope-labeled internal standards enhances quantitative accuracy at parts-per-billion (ppb) levels.
How to order?







