Pharmaceutical Industry
-
Comprehensive transcriptomic and proteomic analysis under different experimental conditions
-
Identification of differentially expressed genes and proteins related to disease mechanisms
-
Pathway mapping and potential drug target identification
-
Correlation analysis between molecular expression levels and phenotypic characteristics
-
Identification of novel disease targets and signaling pathways
-
Target regulation and mechanism validation
-
Comparative molecular analysis between healthy and diseased models
-
Discovery of molecular biomarkers for drug efficacy prediction
-
Quantitative proteomic analysis under drug treatment or disease conditions
-
Targeted validation of candidate biomarkers
-
Functional annotation and pathway enrichment analysis of differential proteins
-
Correlation analysis between proteomic data and pharmacodynamic indicators
-
Discovery of biomarkers related to efficacy or diagnosis
-
Analysis of drug–target interaction mechanisms
-
Study of therapeutic response and resistance pathways
-
Qualitative and quantitative analysis of parent drugs and metabolites
-
In vitro and in vivo metabolic stability testing
-
Evaluation of drug–drug interactions and enzyme induction
-
Pharmacokinetic profiling and clearance rate analysis
-
Bioavailability and ADME studies
-
Identification of active, intermediate, and toxic metabolites
-
Comparative metabolic analysis across species or formulations
-
Mechanistic study of drug metabolism and toxicity
-
Systematic analysis of phosphorylation, acetylation, ubiquitination, glycosylation, and other modification events
-
Identification of modification sites and motif analysis
-
Functional annotation and regulatory network construction
-
Integration of PTM data with proteomic and metabolomic results
-
Mechanism study of kinase inhibitors and epigenetic modulators
-
Identification of PTM biomarkers related to efficacy or resistance
-
Analysis of drug-induced signaling regulation and feedback mechanisms
-
Study of cellular stress response and functional regulation
-
Purity, impurity profile, and structural identification of active pharmaceutical ingredients
-
Quantitative analysis and stability testing of finished drugs
-
Quantification of residual solvents, degradation products, and impurities
-
Drug release kinetics and consistency evaluation
-
Characterization of solid form, dissolution behavior, and physicochemical properties of formulations
-
Quality control and batch-to-batch consistency evaluation of APIs
-
Physicochemical characterization and stability verification before drug registration
-
Establishment of quality standards for biologics and small-molecule drugs
-
Analytical method development and validation during drug research and development
-
Characterization of primary protein structure and post-translational modifications
-
Evaluation of secondary and tertiary structures
-
Analysis of stability, aggregation, and conformational changes
-
Structural comparison between biosimilars and originator drugs
-
Structural characterization of recombinant proteins and monoclonal antibodies
-
Stability and stress testing of drug formulations
-
Correlation between conformational features and bioactivity
-
Consistency and quality comparison of biopharmaceuticals
MtoZ Biolabs provides comprehensive analytical and research support for pharmaceutical companies, biotechnology firms, and research institutions. From drug target discovery and mechanism of action studies to biopharmaceutical characterization and pharmaceutical analysis, we deliver data-driven solutions that support drug discovery, quality control, and new drug development.
With advanced LC–MS/MS analytical platforms, an experienced scientific team, and standardized experimental workflows, MtoZ Biolabs provides high-precision and reproducible analytical data to support pharmaceutical research and decision-making.
Services at MtoZ Biolabs
1. Drug Target Discovery and Validation
Techniques Used: Quantitative proteomics (TMT/iTRAQ/SILAC), RNA-Seq, integrated bioinformatics analysis
Service Content:
Typical Applications:
Figure 1. Overview of Molecular- and System-based Approaches to Target Discovery
2. Pharmacoproteomics and Biomarker Discovery
Techniques Used: High-resolution LC–MS/MS (Orbitrap Fusion Lumos, Q Exactive HF), label-free quantification, PRM/MRM targeted analysis
Service Content:
Typical Applications:
Cruchaga, C. Cell. 2025.
Figure 2. Identification of Novel Biomarkers and Druggable Targets through Blood Proteomics
3. Drug Metabolism and Pharmacokinetics (DMPK) Analysis
Techniques Used: LC–MS/MS, GC–MS, UHPLC-QTOF-MS, targeted and untargeted metabolomics
Service Content:
Typical Applications:
De, San-Martin, BS. et al. Arch Endocrinol Metab. 2021.
4. Post-Translational Modification (PTM) and Mechanism of Action Studies
Techniques Used: Specific peptide enrichment (IMAC, TiO₂, anti-PTM antibodies), LC–MS/MS, pathway analysis
Service Content:
Typical Applications:
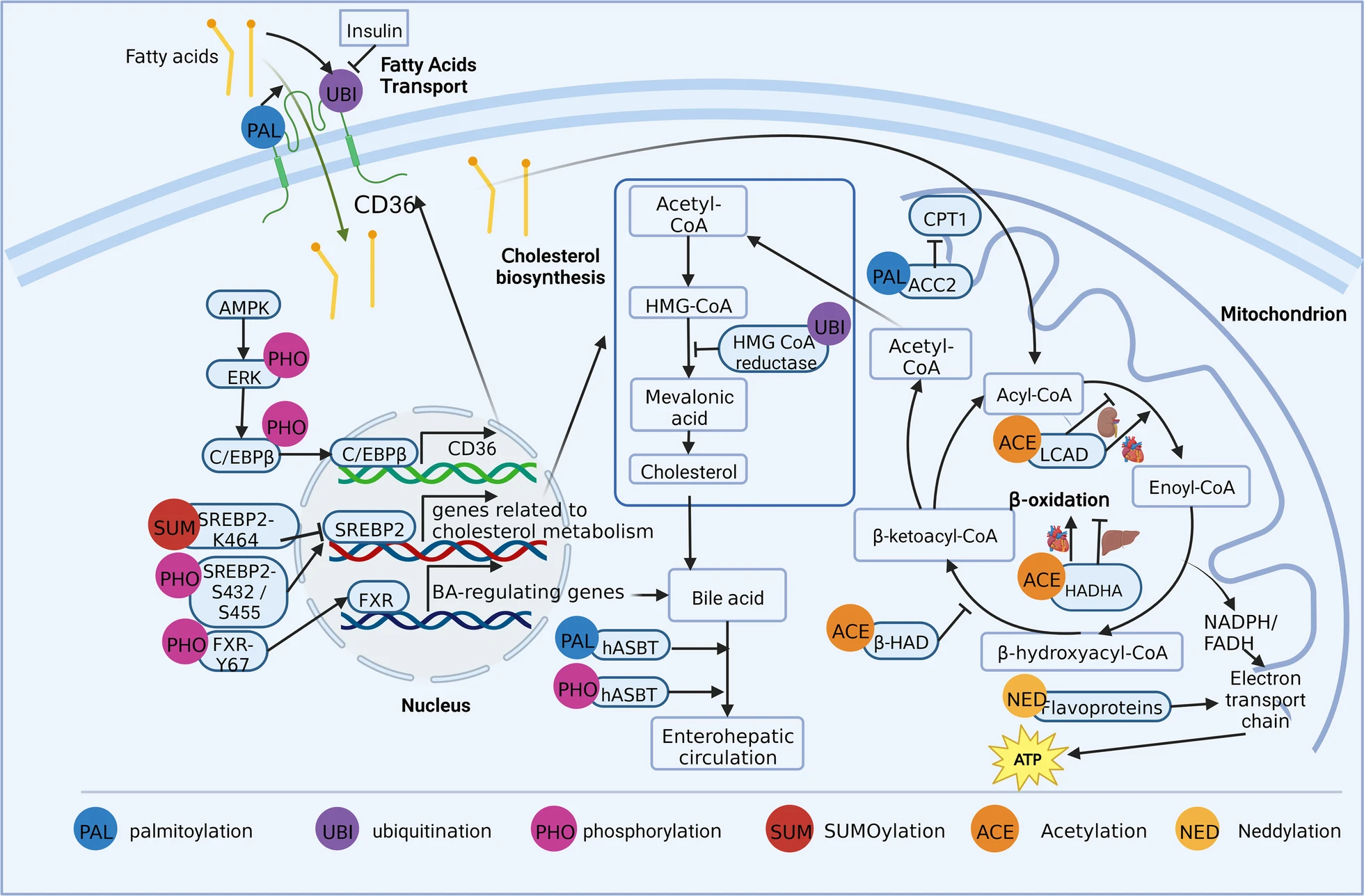
Yang, YH. et al. Mol Med. 2023.
5. Active Pharmaceutical Ingredient (API) and Drug Testing Services
Techniques Used: High-resolution mass spectrometry (LC–MS/MS, GC–MS), HPLC, UPLC, NMR, spectroscopic and structural analysis
Service Content:
Typical Applications:
Chanda, A. et al. Org Process Res Dev. 2015.
Figure 5. API Process Workflow and Typical Analytical Tools
MtoZ Biolabs integrates mass spectrometry, chromatography, and spectroscopy platforms to provide complete quality testing services from API to final formulation, ensuring compliance with international research and regulatory requirements.
6. Biopharmaceutical Structural and Biophysical Characterization
Techniques Used: LC–MS peptide mapping, intact mass analysis, circular dichroism (CD), dynamic light scattering (DLS), X-ray diffraction (XRD), cryo-electron microscopy (Cryo-EM)
Service Content:
Typical Applications:
Si, D. et al. WIRES COMPUT MOL SCI. 2021.
Figure 6. Schematic Diagram of Cryo-EM De Novo Modeling Workflow
Sample Handling and Quality Assurance
To ensure data accuracy and reproducibility, MtoZ Biolabs implements strict quality control procedures throughout sample preparation, testing, and data analysis.
🔹 Accepted Sample Types: Cell lines, tissue samples, serum or plasma, purified proteins, biopharmaceuticals, and metabolite extracts.
🔹 Recommended Storage: Snap-freeze samples in liquid nitrogen and store at −80 °C to avoid repeated freeze–thaw cycles.
🔹 Quality Check Items: Assessment of sample purity, integrity, and concentration.
🔹 Quality Assurance Measures: Use of internal standards, reference materials, and instrument calibration procedures to ensure traceable and consistent results.
Why Choose MtoZ Biolabs
✅ Multi-platform Integration: Comprehensive coverage of proteomics, metabolomics, transcriptomics, and structural analysis to support systematic research.
✅ International Standard Workflows: Compliant with global pharmaceutical research and data integrity standards.
✅ Experienced Scientific Team: Interdisciplinary experts with backgrounds in drug discovery, analytical chemistry, and pharmaceutical development.
✅ Flexible Customized Solutions: Experimental design and reporting tailored to specific research objectives.
✅ High-Value Deliverables: Provision of raw data, quantitative results, pathway maps, and professional research reports supporting publication and regulatory submission.
Start Your Project with MtoZ Biolabs
MtoZ Biolabs is committed to advancing global pharmaceutical innovation through state-of-the-art analytical science and multi-omics technologies. Whether your research focuses on target discovery, mechanism studies, or testing of active pharmaceutical ingredients and biopharmaceuticals, we provide comprehensive support from experimental design to data interpretation.
Contact MtoZ Biolabs to learn more about our Pharmaceutical Industry Research Services and obtain customized project solutions to accelerate your drug development goals.
How to order?







