Exosome In Vivo Functional Research Services
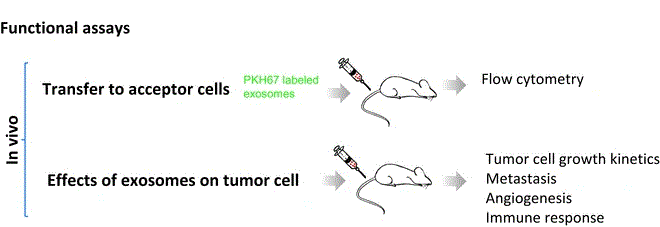
Exosome In Vivo Functional Research Services are designed to investigate the in vivo functions and mechanisms of natural or engineered exosomes using animal models. This comprehensive service includes experimental modules such as fluorescence/bioluminescence tracking, pharmacokinetics analysis, organ distribution, targeting validation, therapeutic efficacy assessment, and toxicological evaluation. Together, these analyses offer a complete view of the biological behavior, functional effects, and therapeutic potential of exosomes in vivo.
Exosomes, as critical mediators of intercellular communication, play essential roles in various physiological and pathological processes. They have shown great potential in applications such as tumor metastasis, immune regulation, tissue repair, and drug delivery. Compared to in vitro models, in vivo functional studies provide a more realistic representation of exosome behavior and mechanisms of action within the physiological environment.
Valencia K. Lecanda F. Springer New York. 2016.
In vivo functional studies of exosomes rely on efficient and traceable labeling strategies. Once labeled, exosomes are introduced into animal models via various administration routes—such as intravenous, intraperitoneal, or localized injection. Their biodistribution and dynamic behavior can then be monitored through fluorescence/bioluminescence imaging, magnetic resonance imaging (MRI), or immunohistochemical staining. These observations, combined with phenotypic assessments and tissue/cellular-level validations, allow for in-depth evaluation of exosome functions and mechanisms in targeted delivery, disease intervention, or immune regulation.
MtoZ Biolabs offers Exosome In Vivo Functional Research Services encompassing multi-dimensional analyses of exosome biodistribution, biological functions, targeting ability, therapeutic efficacy, and safety in vivo. This service helps clients systematically understand the mechanisms and therapeutic potential of exosomes, supporting advanced applications in drug development, disease modeling, and mechanistic studies.
Technical Principles
The functions of exosomes in vivo depend on their targeted localization, cargo release, and regulation of target tissue cell behavior:
1. Targeted Homing: Exosomes are enriched with membrane proteins such as integrins and tetraspanins derived from their parent cells, which guide their accumulation at specific tissues or lesion sites.
2. Cargo Delivery: Once internalized by target cells, exosomes release their encapsulated functional molecules, including miRNAs and proteins, to modulate cellular signaling pathways.
3. Biological Function Execution: These interactions can lead to tissue repair, anti-inflammation, immune modulation, anti-tumor effects, or anti-fibrotic responses in vivo.
This process can be validated through the construction of animal models, tracer labeling, behavioral assays, molecular profiling, and histological analysis.
Applications
The specific applications of Exosome In Vivo Functional Research Services include but are not limited to the following:
Biodistribution and Organ Targeting Analysis
Determining tissue-specific accumulation patterns of exosomes to guide therapeutic delivery strategies.
In Vivo Evaluation of Exosome-Drug Delivery Systems
Assessing the efficacy of exosomes loaded with miRNA, siRNA, or small molecule drugs in disease models.
Tumor Therapy and Immune Response Studies
Investigating the roles of exosomes in anti-tumor activity, immune activation, or inflammation suppression.
Functional Assessment of Stem Cell-derived Exosomes
Verifying the regenerative or anti-fibrotic effects of exosomes derived from MSCs, iPSCs, and other stem cells.
Pharmacokinetics and Stability Analysis
Measuring circulation half-life, clearance pathways, and systemic behavior of exosomes in vivo.
Service Advantages
1. Advanced Analysis Platform: MtoZ Biolabs provides a cutting-edge platform for high-quality, fast, and reproducible in vivo exosome evaluation.
2. Multiplexed Labeling and Tracking Capabilities: Supports fluorescence, bioluminescence, and tissue quantification approaches for real-time visualization and targeting validation throughout the exosome life cycle.
3. One-Time-Charge: Transparent pricing with no hidden fees.
4. Comprehensive Functional Assessment System: Integrates biodistribution, targeting, biological function, mechanism analysis, and toxicity testing into a complete in vivo research pipeline. Optional integration with in vitro platforms supports seamless mechanistic-to-application workflows.
FAQ
Q. How to Trace Exosomes and Assess their Biological Effects in Vivo?
We utilize a range of labeling and imaging strategies—such as lipophilic fluorescent dyes, bioluminescent reporter genes, and molecular imaging technologies (e.g., PET, MRI)—to monitor exosome distribution, transport, and cellular uptake. Functional assays are used to assess downstream effects on recipient cells, including changes in behavior, gene expression, and signaling pathways.
Q. Does your Labeling Process Alter the Structure or Function of Exosomes? Do you Validate Post-Labeling Activity?
At MtoZ Biolabs, we prioritize minimal disruption to exosome integrity. For example, lipophilic dyes like DiR are carefully optimized in terms of concentration and incubation time, and free dye is removed via ultrafiltration or SEC to eliminate background noise. In the case of endogenous labeling (e.g., CD63-GFP or GLuc fusions), we perform post-labeling validation of particle size, membrane protein expression, uptake efficiency, and biological activity to ensure that the labeling does not compromise function.
Case Study
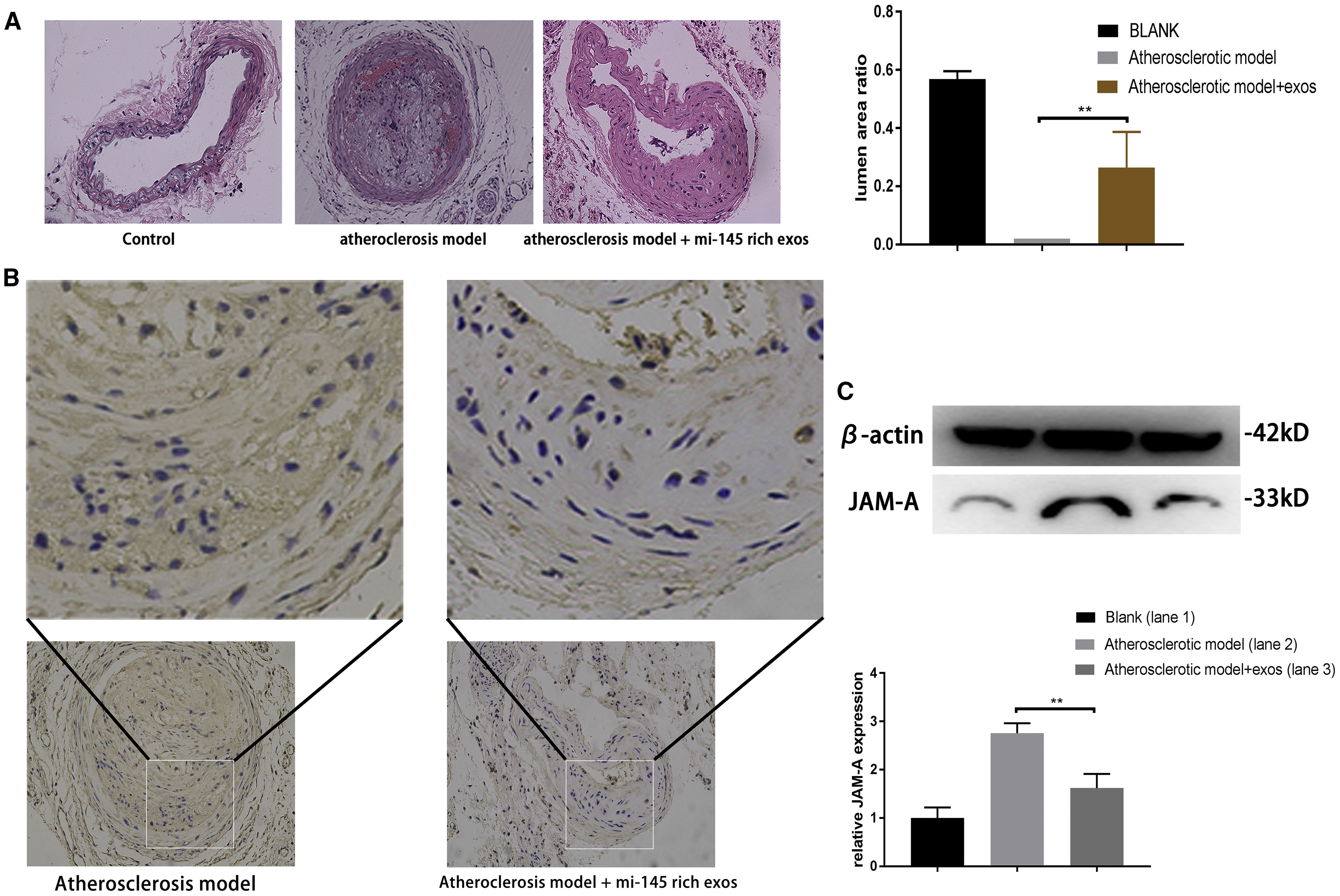
This study employed a comprehensive in vivo functional evaluation strategy to assess the therapeutic role of miR-145-enriched mesenchymal stem cell-derived exosomes (MSC-Exos) in atherosclerosis. Researchers established a high-fat diet-induced mouse model of atherosclerosis and administered miR-145-loaded MSC-Exos via tail vein injection. H&E staining revealed significant reduction in arterial plaque area and lumen expansion in the treated group. Immunohistochemistry (IHC) and Western blot analysis further demonstrated downregulation of JAM-A expression in vascular tissue. The results suggest that miR-145-enriched exosomes inhibit endothelial cell migration via JAM-A suppression, effectively slowing the progression of atherosclerosis in vivo.
Yang W. et al. Mol Ther Nucleic Acids. 2021.
How to order?







