Exosome-Associated Adeno-Associated Virus (AAV) Vector Production Service
- Neutralization by pre-existing antibodies, limiting systemic delivery efficacy;
- Restricted tissue penetration, particularly across the blood–brain barrier (BBB);
- Off-target distribution, resulting in reduced specificity and potential side effects.
- Protect AAV from neutralizing antibodies in circulation;
- Enhance uptake by recipient cells via exosome-mediated endocytosis;
- Improve biodistribution and tissue penetration, particularly in hard-to-access organs like the brain or retina.
- Immune Evasion: Exosomal membranes shield AAV capsids from neutralizing antibodies.
- Enhanced Uptake: Improved cellular internalization via natural exosome pathways.
- Better Tissue Penetration: Facilitates delivery across barriers like the blood–brain barrier.
- Repeat Dosing Potential: Lower immunogenicity enables multiple administrations.
- Customizable Targeting: Exosome surfaces can be engineered for cell-specific delivery.
- Vector Genome Quantification: PCR
- Exosome Profiling: Assessment of size, purity, and concentration
- Biological Function Testing: In vitro and/or in vivo transduction assays
- Safety Checks: Endotoxin level and sterility testing upon request
Gene therapy has rapidly emerged as a transformative approach in the treatment of genetic disorders, cancers, and a wide range of acquired diseases. Among current vector systems, adeno-associated virus (AAV) stands out as one of the most widely used and clinically validated delivery vehicles. AAV vectors offer several advantages, including low immunogenicity, stable gene expression, and broad tropism across dividing and non-dividing cells. Multiple AAV-based gene therapies have received regulatory approval, reinforcing its importance as a cornerstone of modern gene delivery platforms. However, traditional AAV vectors still face critical limitations:
To address these challenges, a promising strategy has emerged—exosome-associated adeno-associated virus vectors (exo-AAVs). Exosome-associated AAVs refer to AAV vector particles that are enclosed within exosomes. During AAV packaging in producer cells, a portion of vector particles become integrated into the exosomal compartment, forming a biologically camouflaged vector system. These hybrid delivery systems harness the biological advantages of exosomes to shield, enhance, and direct AAV delivery in vivo. These hybrid delivery systems:
The combination of natural tropism, immune shielding, and scalable production makes exo-AAV an increasingly valuable option for both experimental gene therapy and clinical vector development.
Service at MtoZ Biolabs
To support cutting-edge gene therapy research and precision delivery development, MtoZ Biolabs offers a specialized Exosome-Associated Adeno-Associated Virus (AAV) Vector Production Service. The Exosome-Associated Adeno-Associated Virus (AAV) Vector Production Service combines our expertise in AAV vector engineering and exosome biology to generate high-quality exo-AAVs optimized for in vitro or in vivo use. Our platform enables researchers to overcome common AAV delivery bottlenecks by producing exosome-enclosed AAV vectors with enhanced immunoevasion, tissue tropism, and delivery efficiency.
We provide the following core services:
✔️Custom AAV Vector Construction: We design and generate AAV vector constructs tailored to your experimental objectives, ensuring that each vector is engineered for high expression efficiency, packaging compatibility, and biological relevance across different AAV serotypes.
✔️Exosome-Associated AAV (exo-AAV) Production: Using optimized transfection and culture conditions, we produce AAV vectors naturally incorporated into exosomes, enhancing delivery efficiency and reducing immunogenicity.
✔️Comprehensive exo-AAV Characterization: We perform rigorous physicochemical and molecular characterization to ensure the integrity and consistency of exo-AAV preparations. Our QC includes nanoparticle size profiling, surface marker validation, quantitative PCR, zeta potential analysis, TEM analysis, and more. These data provide reliable insights into exo-AAV quality and suitability for downstream functional use.
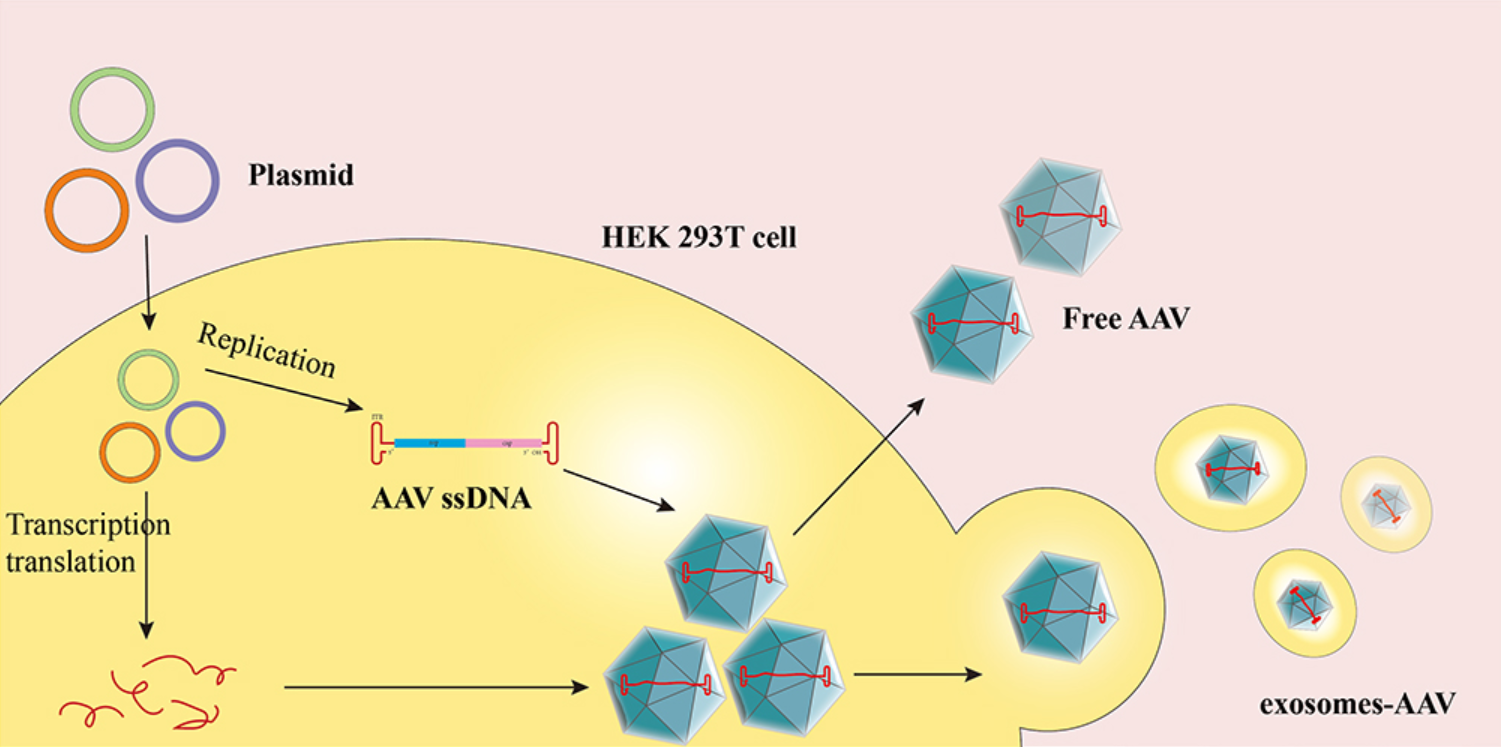
Figure 1. Overview of Exosomes-AAV Production Protocol
Why Choose MtoZ Biolabs?
☑️Integrated Expertise: Specialized in both AAV vector engineering and exosome biology for seamless hybrid vector production.
☑️Flexible Project Design: Customizable vector elements, serotypes, and delivery formats to match your specific therapeutic application.
☑️Rigorous Quality Control: Comprehensive validation of particle size, capsid integrity, exosome markers, and batch-to-batch consistency.
☑️End-to-End Support: From plasmid design to downstream application testing, our team ensures seamless project execution and technical excellence.
Applications
Exosome-associated adeno-associated virus (AAV) vectors are revolutionizing various biomedical fields:
1. Central Nervous System-Targeted Gene Therapy
Enhanced delivery to brain and spinal cord tissues, with improved blood–brain barrier (BBB) penetration and reduced systemic clearance.
2. Immunoevasive Gene Therapy
Exosomal membranes shield AAV capsids from neutralizing antibodies, expanding therapeutic options for patients with pre-existing immunity.
3. Oncology and Tumor Microenvironment Targeting
Tumor-homing exosome-AAV constructs can deliver therapeutic genes directly into cancer cells or stromal components.
4. Precision Medicine Platforms
Engineered exo-AAVs with custom ligands or peptides enable cell-type or tissue-specific targeting for next-generation therapies.
FAQ
Q1: What are the advantages of using exo-AAVs over standard AAVs?
Q2: What quality assurance measures are included?
Exosome-associated AAV vectors represent a powerful next-generation gene delivery strategy, offering enhanced targeting, immune protection, and delivery efficiency. At MtoZ Biolabs, we combine advanced AAV engineering with exosome technology to provide high-quality, customizable Exosome-Associated Adeno-Associated Virus (AAV) Vector Production Service. Contact us to explore tailored solutions for your research.
How to order?







