Exosome-Based Vaccine Development Service
Vaccination remains one of the most effective strategies for preventing infectious diseases, controlling epidemics, and promoting global health. The success of vaccines hinges not only on the antigen design but also on the choice of delivery platform. Traditional vaccine delivery systems include liposomes, polymer-based nanoparticles, viral vectors, and protein subunit carriers—each with its strengths and limitations in terms of stability, targeting, immunogenicity, and safety.
Exosomes have emerged as a promising next-generation delivery system due to their natural biocompatibility, immunomodulatory capabilities, and ability to present antigens in a physiologically relevant context. As nano-sized vesicles secreted by cells, exosomes can be engineered to carry specific antigens, immune-stimulatory molecules, or even adjuvants. This makes them attractive candidates for developing safer and more effective vaccines. MtoZ Biolabs provides comprehensive Exosome-Based Vaccine Development Services supporting researchers and biopharma partners in creating novel vaccine candidates with enhanced targeting, uptake, and immune activation capabilities.
Exosomes offer several compelling benefits as vaccine delivery platforms:
✅Natural origin and biocompatibility: Derived from endogenous cellular pathways, exosomes are inherently non-toxic and non-immunogenic, minimizing the risk of adverse immune reactions.
✅Efficient antigen presentation: Exosomes naturally carry MHC class I and II molecules and other co-stimulatory proteins, facilitating the presentation of antigens to T cells and enhancing adaptive immune responses.
✅Targeted delivery: Surface markers on exosomes allow for tissue-specific targeting, improving vaccine efficacy while reducing off-target effects.
✅Crossing biological barriers: Exosomes can cross blood–brain and other biological barriers, enabling vaccination strategies that target difficult-to-access tissues.
✅Stability and scalability: Exosomes exhibit superior stability under physiological conditions and can be produced at scalable levels using well-established cell culture systems.
Service at MtoZ Biolabs
MtoZ Biolabs offers a full spectrum of customized solutions to support exosome-based vaccine development across preclinical research and translational pipelines. With deep expertise in exosome biology and advanced omics technologies, we provide tailored services to meet the specific needs of vaccine design, validation, and optimization. Our exosome-based vaccine development services include:
💠Exosome Isolation and Purification
High-efficiency ultracentrifugation, size exclusion chromatography, or immunoaffinity-based protocols to obtain high-purity exosomes from various biological sources.
Comprehensive characterization of particle size, concentration, morphology, and purity using techniques such as nanoparticle tracking analysis (NTA), transmission electron microscopy (TEM), and Western blotting.
Quantitative and qualitative analysis of canonical exosomal markers (CD63, CD81, CD9, ALIX, TSG101, HSP70) to verify exosome identity and quality.
Custom loading of antigens, peptides, or immune-stimulatory molecules into or onto exosomes through techniques such as electroporation, incubation, or genetic modification.
💠Multi-Omics Profiling of Exosomes
High-resolution mass spectrometry and sequencing-based analysis of exosomal proteome, lipidome, metabolome, and transcriptome to understand exosome composition and guide rational vaccine design.
💠Exosome Functional Analysis (In Vitro & In Vivo)
Functional validation of engineered exosomes, including cellular uptake, antigen presentation assays, immune cell activation profiling, and animal model evaluation for immunogenicity and efficacy.
By integrating these services, MtoZ Biolabs provides end-to-end support for exosome-based vaccine development. From early-stage vesicle preparation to in vivo immunogenicity studies, our experienced team ensures high-quality data generation and project-specific insight to accelerate your vaccine innovation efforts.
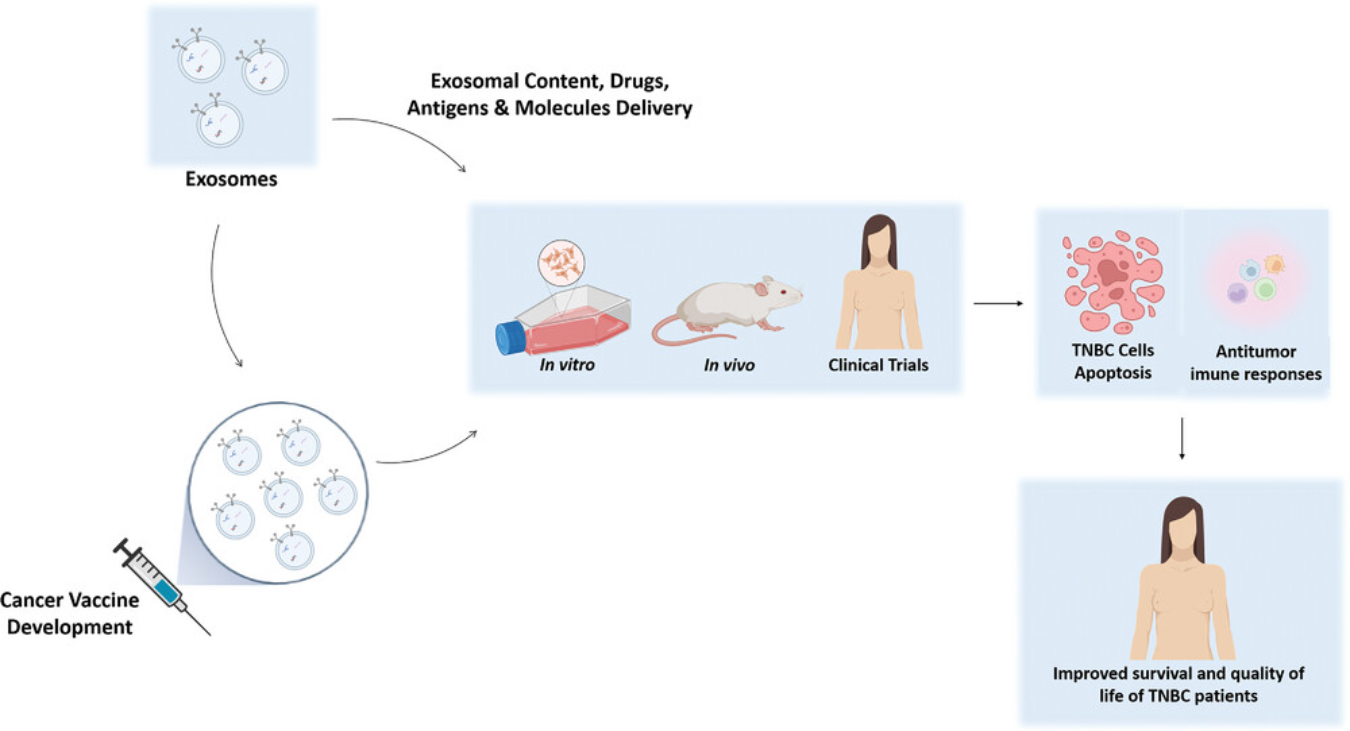
Figure 1. Exosomes-Based Cancer Vaccines to be Used in Triple-Negative Breast Cancer (TNBC) Treatment
Service Advantages
☑️Comprehensive Customization: Tailored workflows designed to meet specific antigen delivery, targeting, and immunogenicity needs.
☑️Advanced Technology Platforms: Integration of high-resolution LC-MS/MS, next-generation sequencing, and nanoparticle tracking systems for accurate characterization and monitoring.
☑️Expertise in Exosome Engineering: Skilled team with deep experience in genetic and chemical modification of exosomes for enhanced vaccine payload delivery.
☑️One-Stop Service: From exosome isolation and engineering to immunogenicity evaluation and functional validation, all procedures are carried out by MtoZ Biolabs, saving clients valuable time and effort.
☑️Strict Quality Control: Rigorous QC at every step ensures consistency, reproducibility, and regulatory compliance.
Sample Submission Suggestions
1. Sample Types
We accept various sample types, including animal tissue, plant tissue, serum/plasma, urine, saliva, CSF, cell culture, and other biological samples.
2. Storage and Shipping Conditions
Samples should be stored at -80℃ and shipped with dry ice. Avoid repeated freeze-thaw cycles.
*Note: If you have specific requirements or need guidance on sample preparation, please do not hesitate to contact us.
Deliverables
1. Comprehensive experimental details (materials, instruments, and methods)
2. Characterization data of exosomes (e.g., size, morphology, surface markers)
3. Vaccine loading efficiency and exosome engineering validation results
4. Multi-omics profiling data (proteomics, lipidomics, metabolomics, transcriptomics, if applicable)
5. Functional assay results (in vitro and/or in vivo, based on project scope)
6. Raw and processed data files (e.g., MS raw data, sequence data, statistical tables)
7. Customized report tailored to project objectives
MtoZ Biolabs is committed to advancing exosome-based vaccine development through customizable solutions. Contact us today to discuss your project. Our technical specialists are available to provide a free business assessment.
Related Services
Exosome Characterization Service
How to order?







