WB-Based Exosome Verification Service
Western blotting (WB) remains a cornerstone technique for protein detection, and is widely recognized in the field of extracellular vesicle (EV) research for confirming the presence of specific exosomal markers. Exosomes—nanoscale vesicles secreted through the fusion of multivesicular bodies (MVBs) with the plasma membrane—exhibit a distinct protein signature, including tetraspanins (CD9, CD63, CD81), membrane trafficking proteins (Alix, TSG101), and stress response proteins (HSP70, HSP90). These biomarkers are essential for exosome identification and provide molecular insights into their origin, function, and biological relevance.
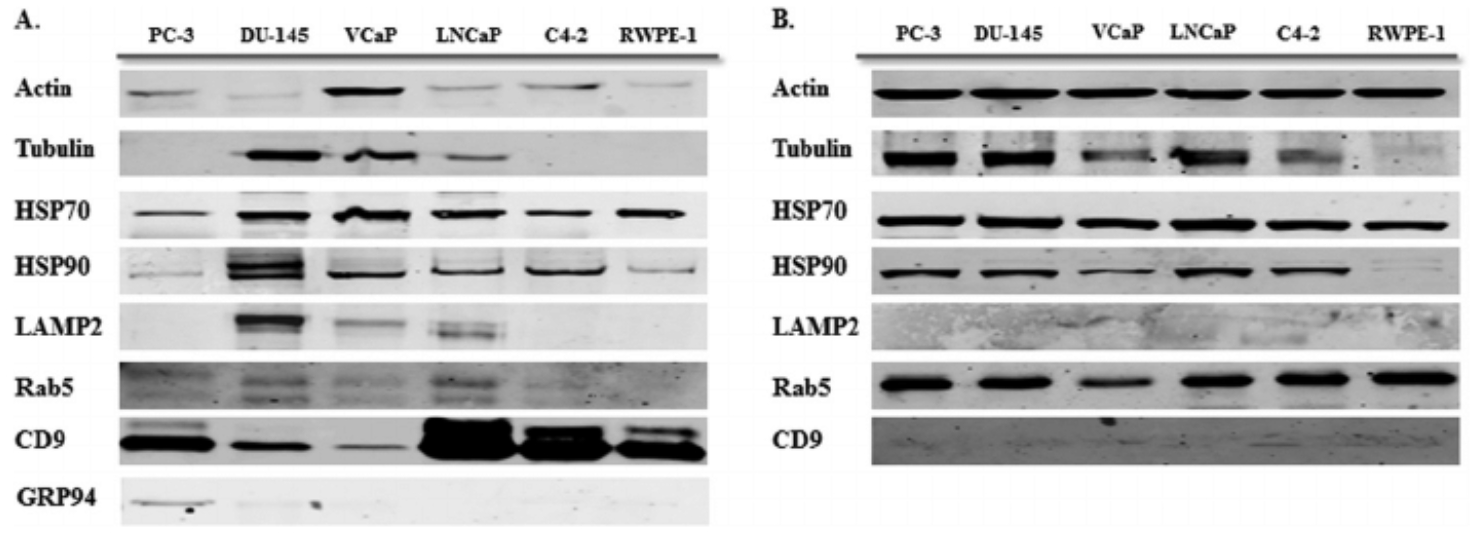
Hosseini-Beheshti, E. et al. Mol. Cell. Proteomics. 2012.
Figure 1. Western Blot Validation of Exosomal Protein Markers
MtoZ Biolabs offers WB-Based Exosome Verification Service for consistent and reliable exosome characterization. By considering sample type and research needs, we offer integrated support from exosome isolation to WB-based validation. This service is applicable to exosomes isolated from various biological sources including cell culture media, plasma, urine, saliva, breast milk, and tissues, facilitating downstream applications such as characterization, quality control, and functional studies.
Analysis Workflow
To ensure consistent and publishable results, our WB-Based Exosome Verification Service follows a fully regulated and reproducible workflow:
1. Exosome Isolation (Optional)
Isolation using ultracentrifugation, membrane filtration, or immunoaffinity capture, customized to your sample type and research needs.
2. Protein Extraction and Quantification
Total protein is extracted from isolated exosomes and quantified using BCA or Bradford assays to ensure accuracy and consistency.
3. SDS-PAGE and Membrane Transfer
Proteins are separated via SDS-PAGE and transferred to PVDF or nitrocellulose membranes for optimal signal retention.
4. Immunoblot Detection
Target markers are detected using client-specified or validated primary antibodies and HRP-conjugated secondary antibodies, with chemiluminescent visualization.
5. Image Generation and Data Interpretation
High-resolution imaging and optional densitometric analysis deliver clear and quantitative insights into exosome protein profiles.
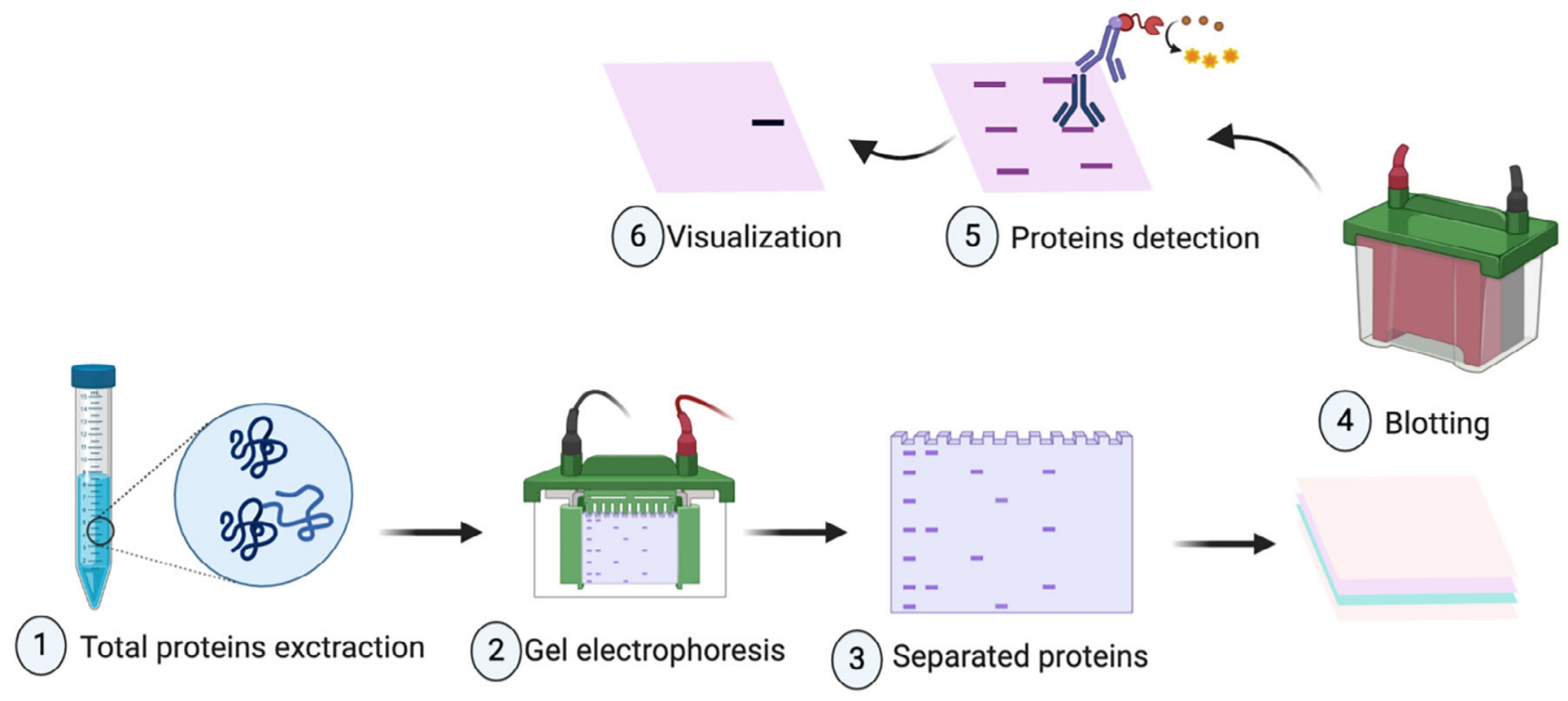
Meftahi, G. H. et al. Biochem Mol Biol Educ. 2021.
Figure 2. Workflow of WB-Based Exosome Verification
Service Advantages
1. ISEV-Guided Marker Selection for Confident Validation
Our workflow aligns with ISEV guidelines, including detection of classical (e.g., CD63, TSG101, Alix, HSP70) and optional project-specific markers to support credible exosome identification.
2. Standardized and Traceable Experimental Workflow
All steps—from protein extraction to signal capture—are carried out under optimized protocols to ensure consistency, minimize batch effects, and support reproducibility.
3. Flexible Service Options from Isolation to Detection
Whether you need end-to-end support or validation-only service, our modular workflow adapts to your project scope while helping reduce hands-on time and resource use.
4. Broad Compatibility with Common Sample Types
Compatible with a variety of biological matrices, including cell culture media, plasma, milk, urine, and saliva—suitable for both research and translational applications.
Sample Submission Suggestions
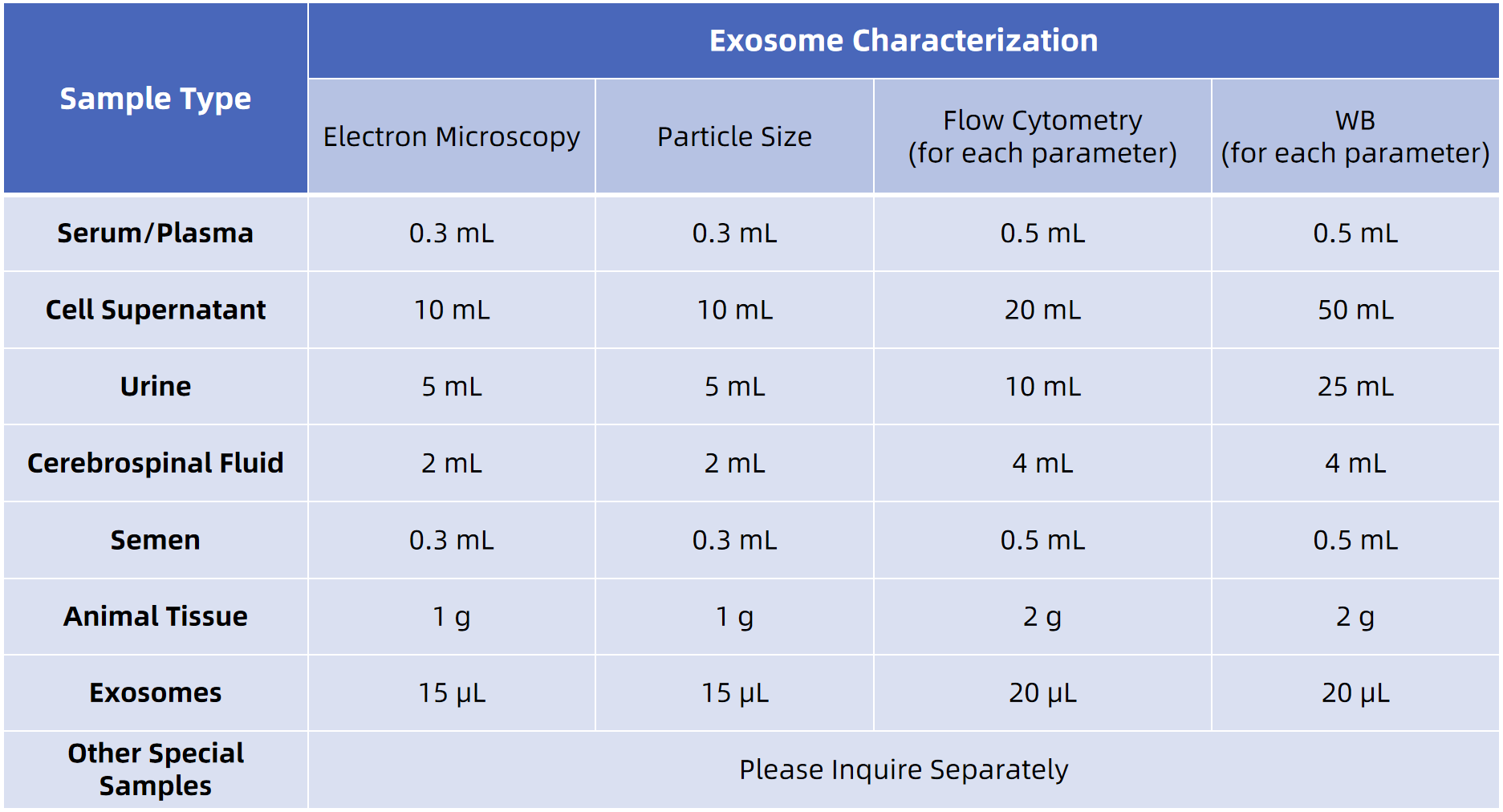
MtoZ Biolabs accepts a wide variety of sample types and offers optional exosome isolation. Detailed submission protocols are available for cell-derived or biofluid samples. For optimal results, we recommend pre-submission consultation with our technical support team to ensure sample compatibility and tailored workflow design.
FAQ
Q1: Do exosomal marker expression levels vary under different conditions?
Yes. Factors such as cell type, culture conditions, and external stimuli can influence the expression of exosomal proteins. We encourage clients to provide relevant background information to optimize marker selection and interpretation.
Q2: Which markers can be detected, and can WB assess exosome purity?
Commonly detectable proteins include tetraspanins (CD9, CD63, CD81), endosomal markers (TSG101, Alix), and HSPs (HSP70). To assess sample purity, we also offer optional detection of negative markers such as Calnexin (ER), GM130 (Golgi), and Histone H3 (nucleus), which help rule out contamination and validate exosome isolation quality.
Protein-level validation is a critical component of exosome research, ensuring accurate identification and supporting the integrity of downstream analyses in areas such as biomarker discovery, therapeutic development, and intercellular communication studies. Integrating established markers with standardized workflows, our approach aligns with current best practices in extracellular vesicle characterization.
MtoZ Biolabs' WB-Based Exosome Verification Service combines technical rigor with application flexibility—offering reliable results, high-quality deliverables, and seamless integration with complementary services like TEM and NTA. Partner with us to enhance the clarity, consistency, and impact of your exosome research.
Related Services
Exosomes Identification Service
Exosome Separation & Purification Service
Transmission Electron Microscope (TEM) based Exosome Characterization Service
Nanoparticle Tracking Analysis-based Exosome Characterization Service
How to order?







