Automated LC-MS-Based High-Throughput Glycan Screening Solution
Glycan modifications play vital roles in numerous biological processes, including cell communication, immune regulation, molecular recognition, and disease progression. Their structural complexity and diversity present significant challenges for both scientific research and industrial applications. A high-sensitivity, high-throughput, and reproducible analytical platform enables the rapid characterization of glycan composition, modification patterns, and functional attributes across diverse sample types, delivering critical data for disease research, drug development, and biomarker discovery.
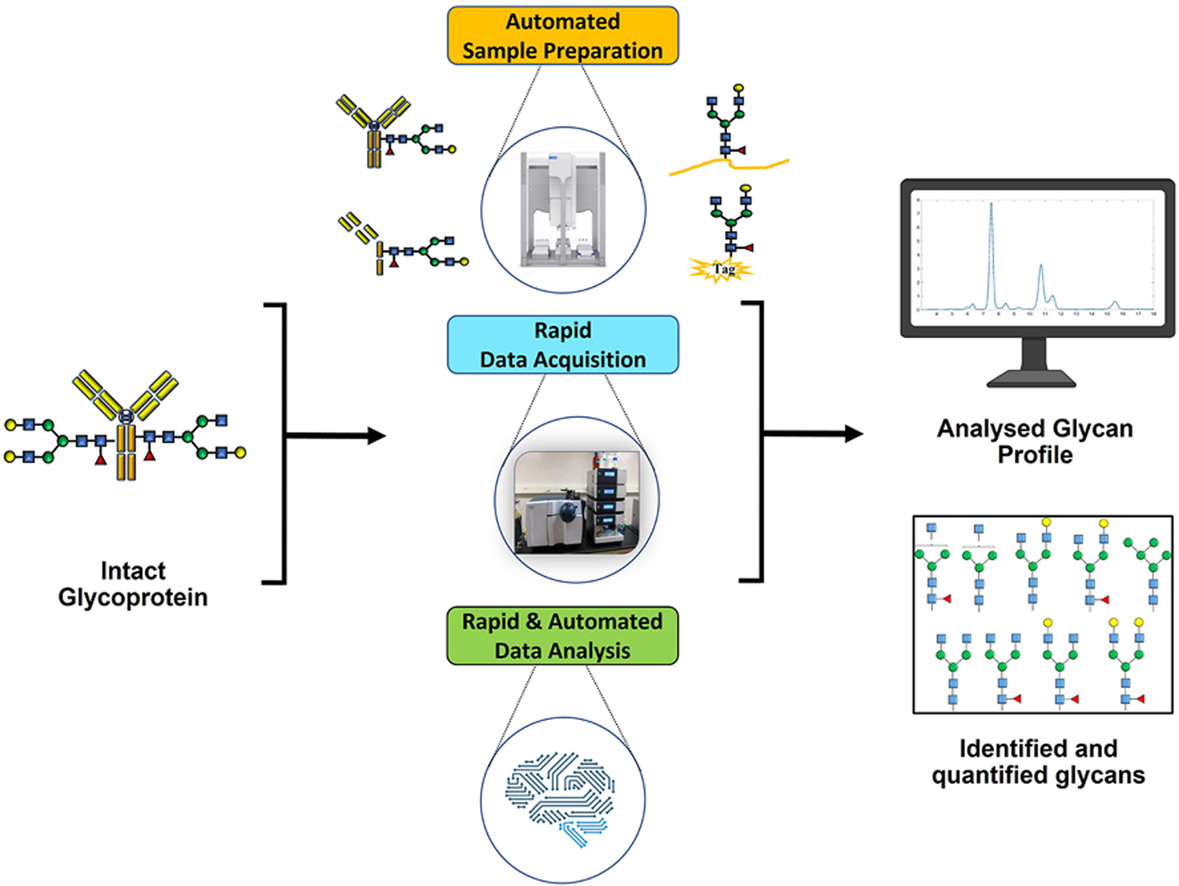
MtoZ Biolabs’ Automated LC-MS-Based High-Throughput Glycan Screening Solution integrates automated sample injection, liquid chromatography (LC) separation, mass spectrometry (MS) detection, and data analysis into a fully streamlined workflow. This closed-loop system increases analytical throughput without compromising precision, minimizes variability introduced by manual handling, and ensures that researchers can obtain reliable glycan data with speed and consistency.
Technical Principles
The automated LC-MS-based high-throughput glycan screening system combines an autosampler, LC separation, and high-resolution MS detection, supported by advanced automation control and dedicated data processing software to maintain precision and efficiency throughout the analysis.
· Liquid Chromatography (LC): Achieves high-resolution separation of glycans based on physicochemical properties such as polarity and molecular size, producing purified fractions for downstream MS analysis.
· Mass Spectrometry (MS): Utilizes highly sensitive and selective acquisition modes to determine glycan molecular weights with high accuracy and to profile structural characteristics.
· Automation Software: Oversees every stage from sample loading to data processing, significantly reducing human intervention and enhancing reproducibility.
By integrating curated glycan databases with advanced computational algorithms, Automated LC-MS-Based High-Throughput Glycan Screening Solution rapidly identifies glycan structures and modification sites. Where necessary, complementary methods such as nuclear magnetic resonance (NMR) can be applied for structural confirmation.
Analysis Workflow
1. Sample Pretreatment
Remove proteins, salts, and other interfering substances to ensure sample purity and LC-MS compatibility.
2. Sample Injection and LC Separation
Load pretreated samples into the LC system, selecting optimal column chemistries and gradient conditions for high-resolution separation of glycans.
3. Mass Spectrometry Detection
Introduce separated glycans into the MS instrument, applying optimized ionization and detection settings (e.g., ESI, TOF) to acquire high-accuracy mass spectra.
4. Data Acquisition and Processing
Use specialized software for peak extraction, signal normalization, background subtraction, and preliminary structure matching.
5. Structural and Modification Analysis
Compare processed spectra against glycan databases to identify structural motifs and modification sites; validate critical findings using complementary techniques such as NMR.
6. Result Integration and Reporting
Deliver standardized reports containing structural assignments, modification annotations, and visual glycan mapping.
Rathore, A. S. et al. Trends Anal. Chem. 2023.
Figure 1. Automated LC-MS Workflow for High-Throughput Glycan Screening
Sample Submission Suggestions
Automated LC-MS-Based High-Throughput Glycan Screening Solution accommodates a wide range of biological materials, including glycoproteins, glycolipids, cell lysates, tissue homogenates, blood, serum, urine, and other biological fluids. Samples may be submitted as liquids or lyophilized powders. To ensure data quality, avoid high-salt content, glycerol, or components with strong UV absorbance.
For complex or unconventional matrices, we recommend consulting the MtoZ Biolabs technical team prior to submission to confirm the pretreatment strategy. A detailed sample preparation and submission guide is available upon request.
Why Choose MtoZ Biolabs?
✅ State-of-the-Art Automated Platform: Fully integrates sample handling, LC separation, and high-resolution MS to deliver high-throughput, precision glycan analysis.
✅ Extensive Glycomics Expertise: Proven experience across basic research, biopharmaceutical development, and clinical translation projects.
✅ Advanced Data Processing Capability: Equipped with dedicated glycan databases and computational tools for structure identification, modification annotation, and visualized reporting.
✅ Broad Sample Compatibility: Supports diverse biological and biochemical sample types, including complex matrices.
✅ Customized Technical Support: Tailors experimental design and data interpretation to project-specific objectives, ensuring both scientific rigor and application relevance.
Applications
● Glycan Modification Mechanism Studies: Elucidate structural patterns and functional changes across different biological states.
● Biopharmaceutical Quality Control: Assess glycosylation consistency in antibodies, recombinant proteins, and other biologics.
● Vaccine Design and Development: Investigate glycan involvement in immune responses to glycan-based vaccines.
● Food and Nutrition Science: Characterize glycan composition and metabolic pathways in food products.
● Drug Research and Optimization: Leverage glycan attributes to enhance drug stability and in vivo distribution.
What Could be Included in the Report?
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Total Ion Chromatogram & Quality Control Assessment
4. Data Analysis, Preprocessing, and Estimation
5. Bioinformatics Analysis
6. Raw Data Files
MtoZ Biolabs’ Automated LC-MS-Based High-Throughput Glycan Screening Solution combines automated sample handling, high-resolution separation, and precision MS detection to deliver large-scale glycan profiling with exceptional speed, reliability, and reproducibility. Whether for fundamental research, clinical investigation, or biopharmaceutical development, we provide robust data support and tailored solutions. Contact us for a customized analytical plan and service quotation.
FAQ
Q1: What is the typical turnaround time?
In most cases, results are delivered within 1–2 weeks after sample receipt, depending on sample number and analytical requirements.
Related Services
High-Throughput Glycan Screening Solution
HPLC-Based High-Throughput Glycan Screening Solution
UHPLC-FLD-Based High-Throughput Glycan Screening Solution
CE-MS-Based High-Throughput Glycan Screening Solution
ESI-MSn-Based High-Throughput Glycan Screening Solution
MALDI-TOF-MS-Based High-Throughput Glycan Screening Solution
How to order?







