New Polypeptide Prodrugs Development Service
Polypeptide prodrugs represent a specialized class of therapeutic compounds in which bioactive drug molecules are chemically conjugated with polypeptides to enhance their pharmacokinetic behavior, biological stability, and tissue-specific delivery. This design enables controlled activation of the drug in vivo, improving therapeutic efficacy while reducing systemic toxicity and dosing frequency. The polypeptide component can function either as the active drug itself or as a biocompatible carrier for a small molecule payload. In both cases, the prodrug is engineered to undergo site-specific or condition-responsive activation, typically through enzymatic cleavage or environmental triggers such as pH or redox gradients. This strategy holds considerable promise for overcoming major limitations associated with conventional peptide drugs, including rapid degradation, poor membrane permeability, and immunogenicity. In addition, polypeptide prodrugs can be tailored for targeted delivery to tumors, inflamed tissues, or other disease-specific microenvironments by incorporating cleavable linkers and targeting ligands.
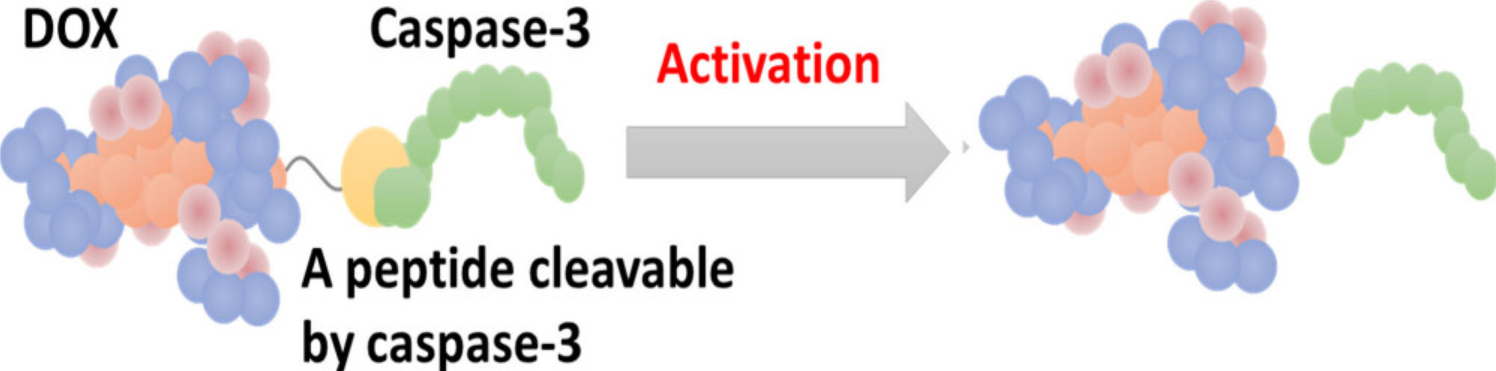
Figure 1. Peptide-Based Prodrug DEVD-S-DOX Activates through Apoptosis Induction by Caspase-3 Expression
Service at MtoZ Biolabs
MtoZ Biolabs specializes in providing integrated New Polypeptide Prodrugs Development Services to design, synthesize, and evaluate prodrugs that enhance the performance of polypeptide-based therapeutics. By leveraging advanced bioconjugation technologies, analytical techniques, and expertise in drug design, we help clients develop innovative polypeptide prodrugs that overcome the challenges of traditional peptide therapies. Our service provides critical support throughout the development process, from initial design to preclinical validation, ensuring that your prodrug candidate reaches its full potential.
💠Polypeptide Prodrug Design and Synthesis
We assist in the design and synthesis of novel polypeptide prodrugs by modifying therapeutic peptides or conjugating small molecules with peptide carriers. This service includes the selection of appropriate linkers, carriers, and bioactive compounds to ensure the prodrug's stability, bioavailability, and activation in the target environment.
💠Physicochemical Characterization
We perform essential physicochemical analyses of polypeptide prodrugs, including molecular weight, solubility, charge distribution, and hydrophobicity. We also use NMR for structural analysis, CD for conformation studies, DLS to assess aggregation, and SPR for molecular interaction evaluation. These analyses are critical for understanding the prodrug's stability and behavior in biological environments.
💠Bioactivity Evaluation
Using in vitro assays, we evaluate the biological activity and efficacy of polypeptide prodrugs. This includes testing their ability to exert therapeutic effects, such as receptor binding, enzyme inhibition, or cytotoxicity against target cells, to ensure that the prodrug exhibits the desired bioactivity once activated.
We conduct pharmacokinetic studies to assess the absorption, distribution, metabolism, and excretion (ADME) properties of the polypeptide prodrug. These studies help determine the optimal dosage, frequency, and delivery method, as well as predict the drug's behavior in clinical settings.
💠Stability and Formulation Optimization
To ensure the long-term stability of the polypeptide prodrug, we perform stress testing under various conditions (e.g., temperature, light, and pH). Our team also works to optimize drug formulations, enhancing stability, solubility, and delivery characteristics for improved therapeutic outcomes.
💠Toxicity and Safety Assessment
We assess the safety and toxicity profile of polypeptide prodrugs through both in vitro and in vivo studies. This includes evaluating cytotoxicity, hemolysis, immunogenicity, and overall biocompatibility to ensure that the prodrug is safe for clinical applications.
Analysis Workflow
1. Target Identification: Identify the biological target for therapeutic intervention.
2. Polypeptide Design: Design the polypeptide sequence to interact effectively with the target.
3. Polypeptide Synthesis: Synthesize the designed polypeptide using solid-phase or solution-phase methods.
4. Prodrug Modification: Modify the polypeptide to create a prodrug with enhanced drug-like properties.
5. In Vitro Testing: Conduct initial in vitro screenings to assess biological activity and prodrug conversion.
6. Pharmacokinetic and Stability Testing: Evaluate absorption, distribution, metabolism, and excretion (ADME), as well as stability under various conditions.
7. In Vivo Efficacy: Test the prodrug's therapeutic effects in animal models.
8. Optimization: Optimize the prodrug formulation for improved performance and efficacy.
9. Toxicity and Safety Evaluation: Perform toxicity and safety assessments to ensure the prodrug is safe for clinical use.
Service Advantages
☑️Advanced Analysis Platform: MtoZ Biolabs established an advanced New Polypeptide Prodrugs Development Service platform, guaranteeing reliable, fast, and highly accurate analysis service.
☑️Comprehensive End-to-End Support: MtoZ Biolabs provides a complete solution from polypeptide design to preclinical evaluation, ensuring every step of the prodrug development process is expertly managed.
☑️Tailored Solutions: We offer customized development strategies to meet the specific needs of your polypeptide prodrug, optimizing its effectiveness and therapeutic potential.
☑️One-Time-Charge: Our pricing is transparent, no hidden fees or additional costs.
Applications
1. Therapeutic Drug Development
Our service enables the creation of polypeptide prodrugs for improved efficacy and targeting in a wide range of therapeutic areas, including cancer, neurodegenerative diseases, and immune disorders.
2. Enhanced Drug Delivery
Polypeptide prodrugs are designed to overcome limitations in drug delivery, such as poor bioavailability and rapid clearance, enhancing the pharmacokinetic properties of peptide-based therapeutics.
3. Biologics and Gene Therapy
The service supports the development of polypeptide prodrugs in biologics and gene therapy, offering enhanced stability, targeted delivery, and optimized release profiles for complex therapeutic molecules.
4. Agricultural Biotechnology
In plant systems, our service helps design prodrugs to improve crop resilience, stress tolerance, and disease resistance, contributing to advancements in agricultural biotech.
5. Improved Patient Outcomes
By increasing the stability and targeting efficiency of polypeptides, our service helps develop safer and more effective treatments, ultimately improving patient outcomes across a variety of diseases.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Total Ion Chromatogram & Quality Control Assessment
4. Data Analysis, Preprocessing, and Estimation
5. Bioinformatics Analysis
6. Raw Data Files
MtoZ Biolabs provides comprehensive solutions for the design, synthesis, characterization, and validation of innovative polypeptide prodrugs, helping researchers and drug developers translate promising candidates into clinically viable therapeutics. Free project evaluation, welcome to learn more details!
Related Services
Custom Peptide Synthesis Services
Verification Service of Synthetic Peptide Sequence
Peptide Structure Determination Service
How to order?







