Drug Safety Assessment Service
-
Define therapeutic index and optimal safety dosage accurately.
-
Detect and address safety issues early in development.
-
Inform clinical protocol design with data-backed safety guidance.
-
Meet stringent global regulatory expectations and shorten time to market.
-
Preclinical safety studies for innovative drug candidates
-
Immunogenicity evaluation of biosimilars and generics
-
Immunotoxicity assessment during vaccine development
-
Specificity and immune profiling for therapeutic antibodies
-
Toxicity mechanism analysis of protein and biologic drugs
As pharmaceutical innovation accelerates, the urgency of ensuring drug safety has grown in parallel. According to the World Health Organization, adverse drug reactions are responsible for approximately 2 million deaths worldwide each year. In this context, conducting comprehensive and precise drug safety assessment in the early stages of drug development is essential to mitigate risk and safeguard patient health. A well-designed safety evaluation not only helps predict potential toxicities and side effects but also supports rational dose design, indication selection, and clinical protocol optimization.
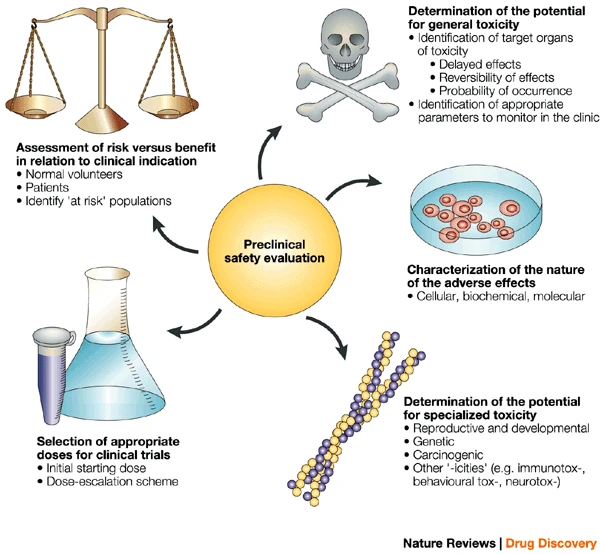
Cavagnaro, J. et al. Nat Rev Drug Discov.
Figure1. Cycle of Preclinical Safety Assessment
What is Drug Safety Assessment?
Drug safety assessment refers to a systematic preclinical evaluation of the toxicological, immunological, and adverse effect profiles of a drug candidate. This process leverages multiple biological and analytical techniques to investigate the compound’s safety window, immune response risk, and potential mechanisms of toxicity. By identifying safety concerns before clinical trials begin, researchers can reduce downstream attrition, improve development efficiency, and optimize cost-effectiveness in the drug development lifecycle.
MtoZ Biolabs Drug Safety Assessment Service
At MtoZ Biolabs, we deliver specialized and data-driven drug safety assessment services to support pharmaceutical R&D. Our platform integrates advanced Immunoaffinity Mass Spectrometry (IMS), high-throughput protein microarrays, and liquid chromatography–tandem mass spectrometry (LC-MS/MS), enabling precise identification of immunogenic risks, off-target effects, and toxic mechanisms. Backed by extensive project experience, we help clients accelerate development while minimizing safety concerns.
Our core service modules include:
✅ Toxicological Profiling
Utilizing both in vitro cellular systems and in vivo animal models, we evaluate the cytotoxic and organ-specific toxicity of drug candidates, providing data to define safety margins and establish NOAEL (No Observed Adverse Effect Level).
✅ Antibody Specificity and Immunogenicity Analysis
Through protein microarray screening and IMS detection, we assess the binding specificity of therapeutic antibodies and evaluate the likelihood of inducing immune responses.
✅ Biomarker-Based Risk Detection
Our LC-MS/MS workflows detect early shifts in safety-relevant biomarkers, enabling mechanism-based insight into toxic side effects and biological perturbations.
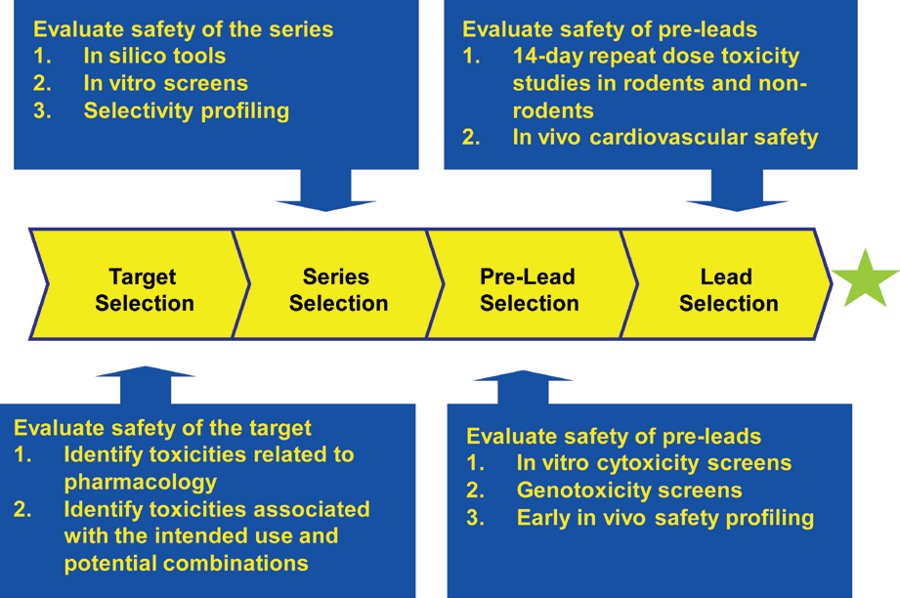
Stevens, G. J. et al. Am. Pharm. Outsourcing.
Figure2. Outline of a Discovery Toxicology Paradigm
Our service helps you:
Applications
MtoZ Biolabs’ drug safety assessment services are applicable to a wide array of drug development programs, including:
Frequently Asked Questions (FAQs)
Q1: How long does it take to complete a full drug safety assessment?
A: Typically, MtoZ Biolabs delivers results within 4–12 weeks, depending on the compound type, number of tests required, and project complexity. Our experienced team optimizes timelines to meet critical development milestones.
Q2: What types of drugs are eligible for your safety assessment services?
A: Our platform supports a broad range of modalities, including small-molecule drugs, biologics (antibodies, recombinant proteins, vaccines), biosimilars, and generics—accommodating various regulatory and clinical-stage needs.
Q3: What sample types and information do I need to provide?
A: Clients are required to submit the test compound and relevant controls, along with detailed information about the drug’s structure, formulation, and prior data (if available). MtoZ Biolabs ensures safe, traceable, and stable sample handling throughout the process.
Q4: What are the advantages of your mass spectrometry-based technologies?
A: We utilize cutting-edge IMS and LC-MS/MS platforms with high specificity and ultra-sensitivity—particularly effective for detecting low-abundance antibodies and biomarkers in complex matrices. These techniques provide accurate insights into potential safety liabilities and mechanistic pathways.
How to order?







