NMR-Based Peptide Structure Analysis Service
- Peptide Drug Structure-Activity Relationship Analysis
- Target Binding Mechanism Research
- Spatial Conformation Screening and Optimization Design
- Natural Bioactive Peptide Structural Function Research
NMR-Based Peptide Structure Analysis Service refers to a professional technical service that uses nuclear magnetic resonance spectroscopy to systematically analyze the structural characteristics of peptide molecules in solution. This service is applicable to a variety of sample types such as natural peptides, synthetic peptides, modified peptides and their complexes. It is widely used in new drug discovery, antibody engineering, enzyme inhibitor development, biomarker screening and other fields, and is an indispensable key support platform for structure-function research.
Peptides, due to their high specificity, biocompatibility, and relatively low toxicity, have shown significant potential in biomedical fields such as new drug development, molecular recognition, signal transduction, and immune regulation. The three-dimensional conformation of peptide molecules plays a decisive role in the realization of their functions. Accurately revealing the three-dimensional structure of peptides is crucial for understanding their structure-activity relationships, pharmacological mechanisms, and target recognition. Nuclear Magnetic Resonance (NMR) is a powerful tool for analyzing molecular structures and dynamic information in solution, with advantages such as not requiring crystals, non-destructive analysis of samples, and the ability to observe dynamic processes. By acquiring multidimensional data, including chemical shifts, coupling constants, and NOE (Nuclear Overhauser Effect), NMR can systematically reveal peptide conformations, flexible regions, spatial arrangements, and interactions with ligands.
Leveraging the NMR platform and multidimensional spectroscopy analysis technology, MtoZ Biolabs provides NMR-Based Peptide Structure Analysis Service for high-resolution analysis of peptide conformational features and spatial structures. This service covers the entire process from one-dimensional, two-dimensional, and three-dimensional spectrum acquisition, chemical shift assignment, NOE constraint extraction, to three-dimensional structure modeling. It is suitable for the structure-function research of natural peptides, synthetic peptides, and their complexes, supporting peptide drug development, target identification, and structure-activity relationship exploration.
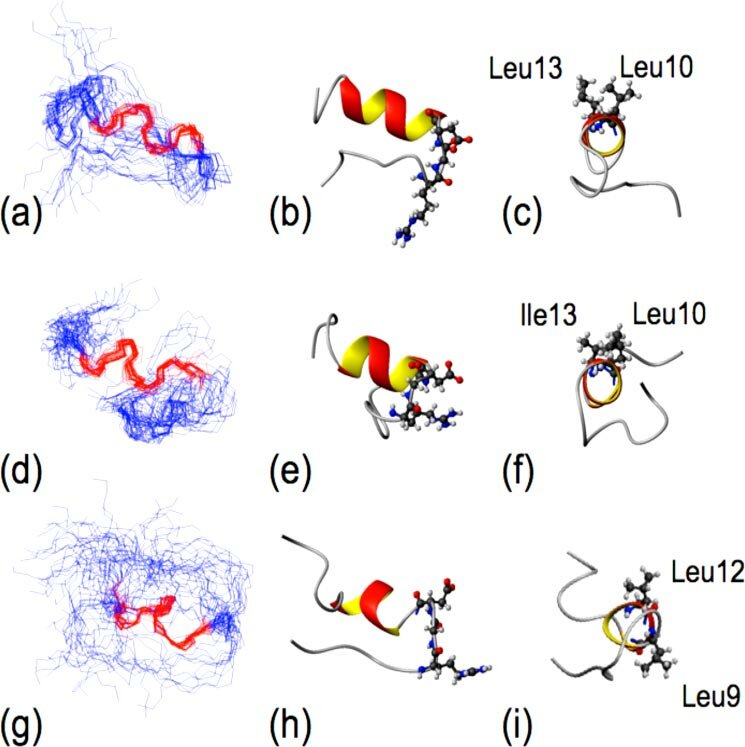
DiCara D. et al. J Biol Chem. 2007.
Figure 1. Analysis of peptide structure using NMR spectroscopy.
Analysis Workflow
The general process of NMR-Based Peptide Structure Analysis Service is as follows:
1. Sample Preparation
The peptide sample undergoes buffer optimization, concentration confirmation, and pH adjustment to ensure it meets the requirements for NMR analysis.
2. NMR Spectrum Acquisition
Based on the sample type and research objectives, a one-dimensional, two-dimensional, or three-dimensional NMR experimental scheme is selected to acquire the necessary spectral data.
3. Chemical Shift Assignment and Data Analysis
The chemical shifts of the main chain and side chain amino acid residues are identified, and the structural domains and potential binding sites are determined.
4. NOE Constraint Acquisition and Structural Calculation
The NOE cross-peak intensities are analyzed to generate distance constraints, which are then input into structural simulation programs for model building.
5. Structure Validation and Report Generation
The preliminary model undergoes energy minimization, secondary structure analysis, and Ramachandran plot validation, with high-quality structural data and a graphical report generated.
Service Advantages
Strong Structural Analysis Capability: Capable of simultaneously acquiring one-dimensional, two-dimensional, and three-dimensional data, supporting a comprehensive analysis from folding state assessment to spatial structure modeling.
Non-destructive Samples, Visualizing Dynamic Information: No crystallization required, allowing for the analysis of the peptide's true spatial conformation and dynamic behavior under near-physiological conditions.
Flexible Experimental Setup: Supports high-resolution analysis of both non-labeled and 15N/13C stable isotope-labeled samples, offering flexibility to meet diverse research needs.
End-to-End Support from Professional Team: Experienced structural biology experts handle data analysis and model building, ensuring scientifically reliable results.
Sample Submission Suggestions
Sample Types: Supports synthetic peptides, naturally sourced peptides, recombinant expressed peptides, etc.
Molecular Weight Range: Recommended to be less than 30 kDa.
Concentration Requirement: Greater than 0.5 mM, with a total volume not less than 450 μL.
Solution Conditions: Use buffers such as PBS or HEPES, with a pH recommended between 6.5 and 7.5. The solution should contain 5-10% D₂O for phase locking and reference signals.
Additional Notes: We recommend contacting us prior to sample submission for detailed and tailored sample preparation guidelines.
Applications
Examples of applications for NMR-Based Peptide Structure Analysis Service:
Uncover the intrinsic link between structural features and pharmacological functions.
Identify the binding regions and conformational changes between peptides and receptors, enzymes, or antibodies.
Guide the structural optimization and stability enhancement of lead peptide molecules.
Analyze the structural foundation of natural peptides derived from animals, plants, or microorganisms.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Total Ion Chromatogram & Quality Control Assessment (project-dependent)
4. Data Analysis, Preprocessing, and Estimation (project-dependent)
5. Raw Data Files
Related Services
How to order?







