Glycoprotein Stable Isotope Quantification Analysis Service
- Characterization of therapeutic glycoproteins to ensure batch consistency.
- Quantitative evaluation of glycosylation for biosimilarity and regulatory compliance.
- Monitoring process-induced changes in glycosylation during production.
- Quantitative identification of glycoproteins as potential disease biomarkers.
- Comparative profiling of clinical samples from patient and control groups.
- Integration of glycoprotein quantification with global proteome studies.
- Evaluation of glycosylation effects on signaling pathways and cellular physiology.
- Assessing the impact of mutations or glycoengineering strategies on glycoprotein abundance and glycosylation.
- Quantitative profiling of glycoproteins in food matrices.
- Study of glycoprotein responses to environmental stresses.
Glycoproteins are vital biomolecules with structural, regulatory, and signaling functions across biology. Their role in immune defense, enzymatic processes, intercellular communication, and therapeutic drug design makes them indispensable targets of modern biomedical research. Glycoprotein Stable Isotope Quantification Analysis involves measuring the abundance of glycosylated peptides or intact glycoproteins through the use of isotopic tracers such as 13C, 15N, 18O, deuterium, or stable isotope-labeled chemical tags. When combined with liquid chromatography–tandem mass spectrometry (LC-MS/MS), this method allows precise quantification of site-specific glycosylation occupancy, glycoform distribution, and structural heterogeneity under different experimental conditions. Compared to conventional label-free methods, stable isotope-based approaches offer higher accuracy, reproducibility, and sensitivity for glycoprotein studies. Such quantitative insights are critical for biopharmaceutical development, biomarker discovery, systems biology, and comparative clinical studies.
Service at MtoZ Biolabs
MtoZ Biolabs offers a complete Glycoprotein Stable Isotope Quantification Analysis Service designed to provide precise quantitative insights into glycosylation. Leveraging advanced isotopic labeling strategies, high-resolution mass spectrometry, and powerful bioinformatics pipelines, our service ensures reliable and reproducible results tailored to client needs.
Our service platform covers a wide range of stable isotope quantification strategies:
💠Metabolic Labeling (e.g., SILAC)
Stable isotope-labeled amino acids are incorporated into proteins during cell culture, enabling accurate relative quantification across different samples.
💠Chemical Labeling (e.g., iTRAQ, TMT)
Isobaric tags carrying stable isotopes are covalently attached to peptides or glycopeptides, allowing multiplexed comparison of up to 16 samples in a single LC-MS run.
💠Enzymatic or In-solution Incorporation
Enzymatic reactions such as trypsin digestion in 18O-labeled water introduce isotopic signatures, providing internal standards for quantification.
💠Spiked Isotopic Standards
Synthetic glycopeptides or proteins with isotopic labeling are introduced into samples as external or internal standards, enabling absolute quantification.
A cost-effective chemical labeling method that offers efficient and accurate relative quantification of glycoproteins in complex mixtures.
Analysis Workflow
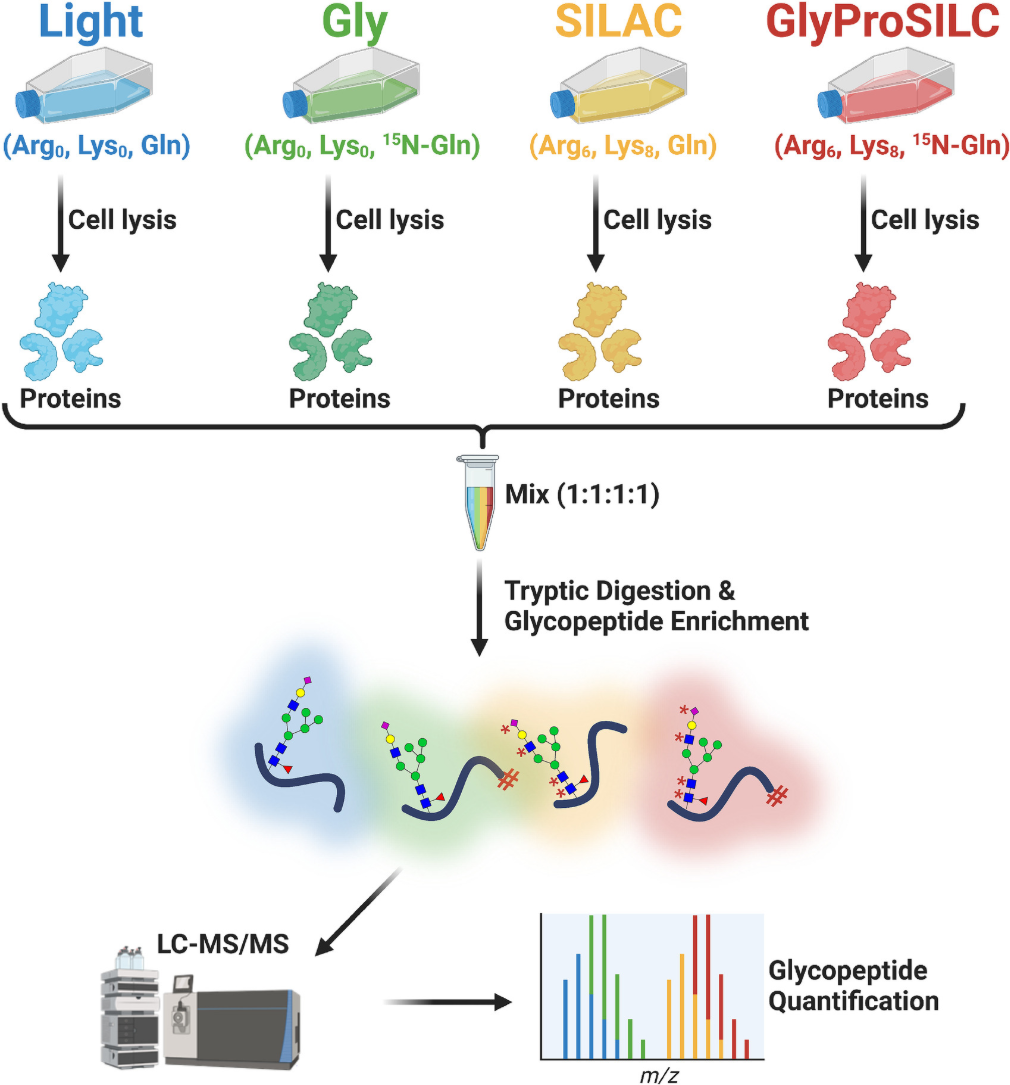
Figure 1. Workflow for Stable Isotope Quantitative LC-MS/MS Glycoproteomics Analysis
Service Advantages
✔️Technical Excellence
Advanced stable isotope labeling strategies combined with state-of-the-art LC-MS/MS instrumentation.
✔️Precision and Accuracy
High reproducibility and low error margins in both relative and absolute quantification.
✔️Integrated Approach
Combining quantification with glycosylation profiling and structural analysis for comprehensive insights.
✔️Customized Solutions
Flexible workflows adapted to specific research or industrial needs.
✔️Expert Support
A professional scientific team guiding every stage of the project, from experimental design to data interpretation.
Applications
1. Biopharmaceutical Development
2. Biomarker Discovery
3. Systems Biology and Proteomics
4. Protein Engineering
5. Food and Environmental Science
Sample Submission Suggestions
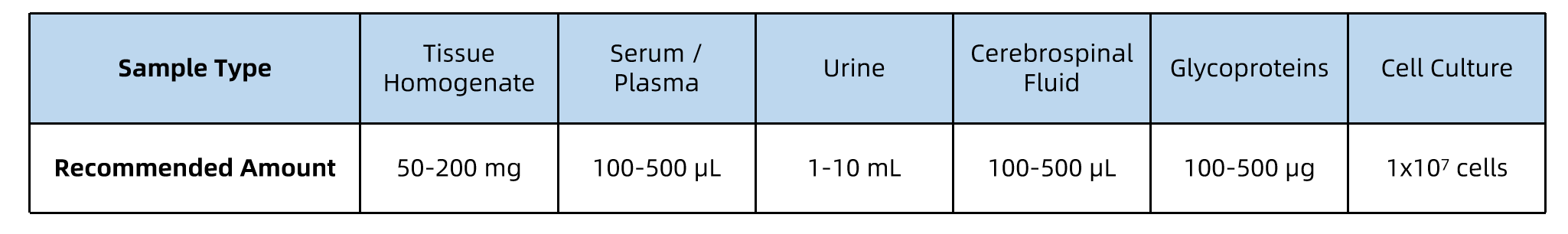
All samples should be kept on ice during processing, and stored at –80°C prior to shipment. Ship samples on dry ice with proper labeling and accompanying documentation.
If you have special sample types or require additional guidance, please contact us for personalized support before sample preparation.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Total Ion Chromatogram & Quality Control Assessment (project-dependent)
4. Data Analysis, Preprocessing, and Estimation (project-dependent)
5. Bioinformatics Analysis
6. Raw Data Files
Related Services
SILAC-Based Quantitative PTM Analysis Service
How to order?







