Glycoprotein Quantification Service
Glycoprotein quantification is the process of accurately measuring the amount of glycoproteins in a sample, either as total glycoprotein content or in the context of specific glycoforms and glycosylation sites. This analytical approach can be applied to purified therapeutic proteins, complex biological fluids such as serum or plasma, cell culture supernatants, and tissue extracts. Glycoprotein quantification provides essential information for both scientific research and industrial applications. In the life sciences, it supports the study of protein function, biomarker discovery, and disease mechanism elucidation. In the biopharmaceutical industry, it plays a critical role in therapeutic protein development, manufacturing, and quality control. For biologic drugs such as monoclonal antibodies, Fc-fusion proteins, and therapeutic enzymes, consistent glycoprotein levels are considered critical quality attributes by regulatory agencies. Accurate quantification helps ensure batch-to-batch consistency, monitor process performance, assess biosimilarity, and maintain product safety and efficacy.
Service at MtoZ Biolabs
MtoZ Biolabs offers a broad portfolio of glycoprotein quantification services designed to meet the diverse needs of both research and industrial applications. Our analytical workflows are adaptable to different sample types and experimental goals, ensuring accurate, reproducible, and meaningful results.
We provide two main types of glycoprotein quantification:
💠Relative Quantification of Glycoproteins
Compares glycoprotein abundance across multiple samples or experimental conditions to determine proportional differences or fold changes. This approach is often used in comparative studies such as treatment evaluation, process optimization, and biomarker discovery. At MtoZ Biolabs, relative quantification is performed using label-free LC-MS/MS analysis or stable isotope labeling techniques such as SILAC, TMT, or iTRAQ, combined with robust chromatographic separation and high-resolution mass spectrometry for accurate glycoprotein quantitation.
💠Absolute Quantification of Glycoproteins
Determines the exact concentration of a glycoprotein in a sample, expressed in absolute units (e.g., ng/mL or µg/mg total protein). This method is essential for applications requiring strict quantitative control, such as therapeutic protein release testing, pharmacokinetic studies, and regulatory submissions. At MtoZ Biolabs, absolute quantification is achieved using mass spectrometry (MS) with synthetic isotopically labeled standards, calibration curves, and multiple reaction monitoring (MRM) or parallel reaction monitoring (PRM) workflows to ensure high specificity and precision.
In addition to glycoprotein quantification, MtoZ Biolabs also provides comprehensive glycoprotein analysis and characterization. This includes glycosylation site mapping, glycoform profiling, glycan structure elucidation, and heterogeneity assessment at both the glycopeptide and intact protein levels. By integrating quantitation with detailed structural characterization, we deliver a complete understanding of glycoprotein composition, distribution, and functional relevance.
Analysis Workflow
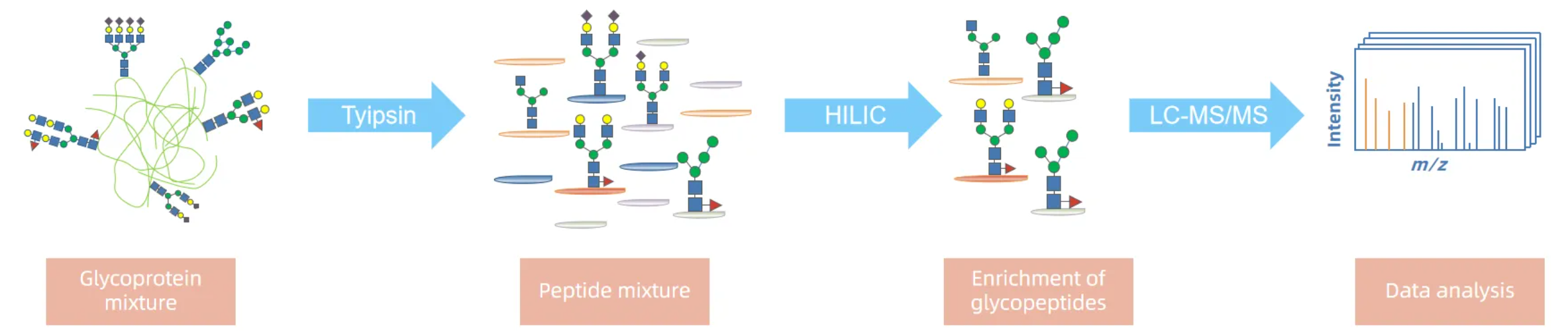
Service Advantages
✔️Advanced Analytical Platforms
MtoZ Biolabs established an advanced Glycoprotein Quantification Service platform, guaranteeing reliable, fast, and highly accurate analysis service.
✔️Innovative Quantification Methods
Utilization of both label-free and isotope-labeled quantitation strategies, combined with optimized enrichment and separation workflows, to deliver reliable results for both relative and absolute glycoprotein measurements.
✔️Expert Scientific Team
A dedicated team of experienced scientists with deep expertise in glycoproteomics, mass spectrometry, and biopharmaceutical analytics ensures high-quality data and insightful interpretation.
✔️Customized Solutions
Flexible, project-specific workflow design to meet unique research goals and regulatory requirements, from early-stage discovery to large-scale quality control.
Applications
1. Biomarker Discovery
Quantitative profiling of glycoproteins in biological fluids and tissues to identify potential diagnostic and prognostic biomarkers.
2. Disease Mechanism Research
Investigation of glycoprotein expression and glycosylation changes associated with pathological processes such as cancer, autoimmune disorders, and infectious diseases.
3. Drug Development
Quantitation of therapeutic glycoproteins, including antibodies, Fc-fusion proteins, and therapeutic enzymes, to support candidate selection, process optimization, and regulatory submissions.
4. Food and Environmental Science
Analysis of glycoprotein content and profiles in food products or environmental samples for quality control, authenticity testing, and contamination assessment.
Sample Submission Suggestions
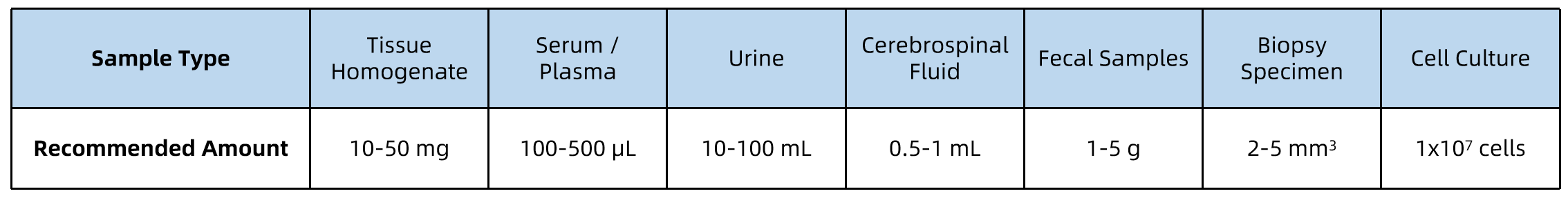
All samples should be collected under sterile conditions, kept on ice during processing, and stored at –80°C prior to shipment. Ship samples on dry ice with proper labeling and accompanying documentation.
If you have special sample types or require additional guidance, please contact us for personalized support before shipping.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Total Ion Chromatogram & Quality Control Assessment (project-dependent)
4. Data Analysis, Preprocessing, and Estimation (project-dependent)
5. Bioinformatics Analysis
6. Raw Data Files
Related Services
Glycoprotein Label-free Quantitative Analysis Service
Glycoprotein Profiling Service
How to order?







