DNA/RNA Drug Purity and Impurity Analysis Service
MtoZ Biolabs provides a dedicated DNA/RNA Drug Purity and Impurity Analysis Service to support the quality assessment of nucleic acid therapeutics. Our service enables precise evaluation of purity levels and comprehensive impurity profiling, ensuring that DNA and RNA drugs meet rigorous scientific and regulatory expectations.
DNA and RNA drugs, including antisense oligonucleotides, siRNA, mRNA, and other nucleic acid-based therapeutics, have become an essential class of modern biopharmaceuticals. Their unique mechanisms of action enable targeted gene regulation, protein expression, and disease intervention. However, as nucleic acids are highly sensitive molecules with complex chemical modifications, ensuring their purity and accurately profiling impurities are critical for guaranteeing drug safety, therapeutic efficacy, and long-term stability.
DNA/RNA drug purity refers to the accurate assessment of intact nucleic acid molecules in a preparation, while impurity analysis focuses on detecting truncated sequences, incomplete syntheses, chemical modifications, and degradation products. These impurities can arise from solid-phase synthesis errors, enzymatic reactions, oxidation, depurination, or contamination introduced during manufacturing and storage. Given that impurities may alter biological activity, trigger immune responses, or compromise stability, detailed characterization is indispensable.
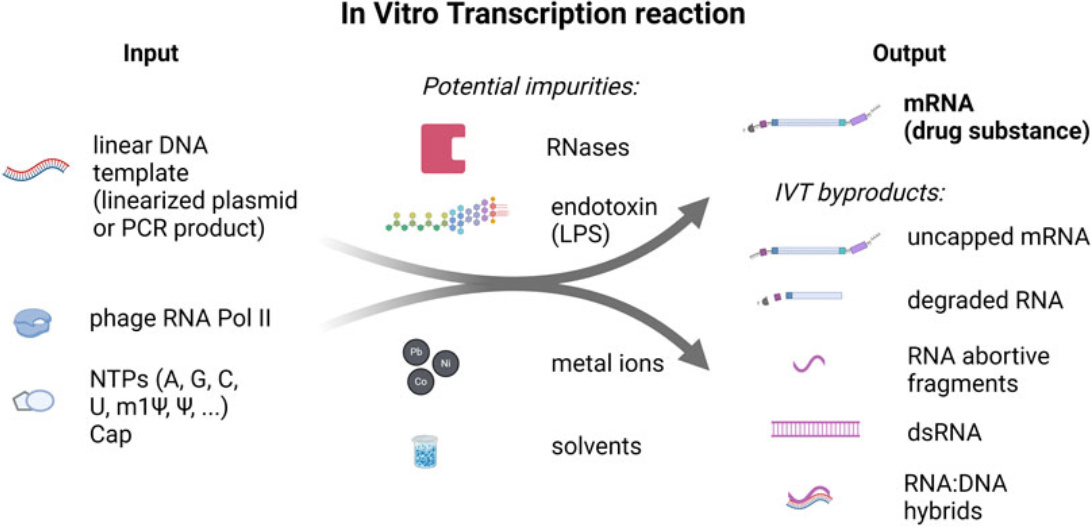
Figure1. In Vitro Transcription Reaction Input and Output Components and Potential Impurities
Service at MtoZ Biolabs
MtoZ Biolabs' DNA/RNA Drug Purity and Impurity Analysis Service is designed to meet the rigorous demands of nucleic acid drug development. Our offerings cover two major areas:
We provide accurate quantification of intact DNA and RNA molecules, ensuring that the therapeutic material meets required specifications. Our workflows allow clear differentiation between full-length products and truncated sequences.
We specialize in the identification and characterization of impurities such as incomplete syntheses, modified nucleotides, degradation products, and contaminants. This analysis supports both process optimization and regulatory compliance.
To achieve reliable and reproducible results, we employ a suite of advanced analytical technologies, including:
🔸High-Performance Liquid Chromatography (HPLC) for separation and quantification of nucleic acid components
🔸Capillary Electrophoresis (CE) for high-resolution impurity profiling based on charge-to-size ratio
🔸Mass Spectrometry (MS) for precise molecular weight determination and structural confirmation
🔸Matrix-Assisted Laser Desorption/Ionization Time-of-Flight (MALDI-TOF) for rapid mass analysis of oligonucleotides
🔸Polyacrylamide Gel Electrophoresis (PAGE) for visualizing purity, size distribution, and integrity
Analysis Workflow
Service Advantages
✔️Cutting-Edge Technology
We employ state-of-the-art platforms including HPLC, CE, MS, MALDI-TOF, and PAGE, ensuring the highest accuracy and resolution in DNA/RNA drug purity and impurity analysis.
✔️Expertise and Experience
Our team brings deep knowledge in nucleic acid chemistry and pharmaceutical analytics, providing reliable solutions backed by years of laboratory experience.
✔️Customized Services
We design flexible workflows tailored to project-specific needs, accommodating diverse sample types, therapeutic formats, and regional regulatory requirements.
✔️Efficiency and Reliability
With streamlined processes and rigorous quality standards, we deliver fast turnaround times without compromising data integrity or reproducibility.
Applications
1. Drug Development and Optimization: Supports candidate optimization by confirming purity and identifying impurities that could affect efficacy or safety.
2. Manufacturing Process Control: Monitors synthesis and purification steps to reduce process-related impurities and ensure consistent product quality.
3. Quality Assurance: Provides validated data packages for regulatory submissions, demonstrating product integrity and safety.
4. Stability Testing: Identifies degradation pathways under stress and storage conditions, guiding formulation and shelf-life studies.
Sample Submission Suggestions
1. Sample Types
Acceptable samples include DNA drugs, RNA drugs, oligonucleotides (modified or unmodified), and aptamers.
2. Storage and Transportation
Samples should be stored at low temperature (-20°C or below), and shipment on dry ice is recommended.
It is recommended to contact the MtoZ Biolabs technical team prior to sample submission to obtain detailed and tailored guidelines for sample preparation and submission.
Deliverables
1. Detailed Experimental Protocols
2. Chromatograms and Electropherograms
3. Mass Spectra and Sequence Confirmation Results
4. Purity Percentages and Impurity Profiles
5. Data Interpretation and Conclusions
6. Raw Data Files
MtoZ Biolabs is committed to providing reliable, comprehensive, and high-quality analytical solutions to support every stage of nucleic acid drug development. Free project evaluation, welcome to learn more details!
Related Services
DNA/RNA Drug Molecular Weight Confirmation Service
How to order?







