DNA/RNA Drug Molecular Weight Confirmation Service
- Sample Types
- Storage and Transportation
DNA/RNA Drug Molecular Weight Confirmation Service is an analytical service established for the structural verification and quality control of DNA and RNA drugs.
In the era of rapid development of nucleic acid therapeutics, DNA/RNA drug molecular weight confirmation has become a critical step to ensure the quality and regulatory compliance of nucleic acid based drugs. Whether it is antisense oligonucleotides (ASOs), small interfering RNA (siRNA), aptamers, or the increasingly prominent mRNA vaccines, the accuracy of molecular weight is directly related to the structural integrity, stability, and clinical efficacy of the drug.
By applying advanced technologies such as high-resolution mass spectrometry (ESI-MS, LC-MS, MALDI-TOF), molecular weight confirmation of DNA and RNA drugs can not only precisely verify whether the synthesized product matches the theoretical sequence, but also detect truncated forms, missing modifications, or degradation products in a timely manner. This provides reliable data support for drug development, quality control, and regulatory submission.
Talhaoui I. et al. J Biol Chem. 2015.
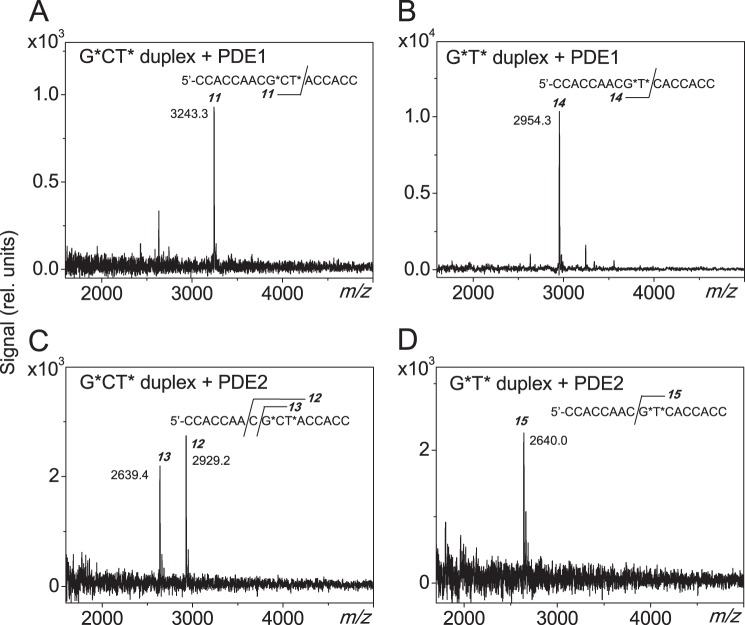
Figure 1. MALDI-TOF/MS analysis of the mixture of oligonucleotides arising from the incubation of the 17-mer G*CT* and G*T* duplexes with phosphodiesterases 1 and 2.
Services at MtoZ Biolabs
Relying on advanced chromatography and mass spectrometry platforms, MtoZ Biolabs provides DNA/RNA Drug Molecular Weight Confirmation Service to achieve accurate measurement and verification of nucleic acid drug molecular weight. Our team has extensive experience in nucleic acid drug analysis and can customize testing strategies based on different modification types and sample characteristics. This enables efficient identification of truncated products, missing modifications, and impurities, supporting comprehensive quality control from basic research to drug development.
MtoZ Biolabs offers multiple mass spectrometry platforms to meet different research needs:
MALDI-TOF Analysis: Enables rapid detection of large DNA and RNA molecules, suitable for drug samples with higher molecular weight.
LC-MS Analysis: Combines liquid-phase separation with mass spectrometry, ideal for complex mixtures, capable of separating different components and identifying impurities and truncated products.
ESI-MS Analysis: Provides gentle ionization and precise molecular weight determination of nucleic acid drugs, particularly suitable for modified samples.
Service Advantages
Advanced Analysis Platform: MtoZ Biolabs established an advanced DNA/RNA Drug Molecular Weight Confirmation Service platform, guaranteeing reliable, fast, and highly accurate analysis service.
One-Time-Charge: Our pricing is transparent, no hidden fees or additional costs.
Professional Data Interpretation: An experienced analytical team can identify truncated products, adducts, and missing modifications to ensure reliable results.
Rigorous Quality Control: Strict experimental standards and data review procedures guarantee accuracy and reproducibility of results.
Customized Solutions: Personalized analytical strategies are provided based on the client’s sample type and research requirements.
Sample Submission Suggestions
Acceptable samples include DNA drugs, RNA drugs, oligonucleotides (modified or unmodified), and aptamers.
Samples should be stored at low temperature (-20°C or below), and shipment on dry ice is recommended.
It is recommended to contact the MtoZ Biolabs technical team prior to sample submission to obtain detailed and tailored guidelines for sample preparation and submission.
Applications
DNA/RNA Drug Molecular Weight Confirmation Service has a wide range of applications, including but not limited to:
Oligonucleotide Synthesis Quality Control
Confirm whether the product meets the designed sequence and modification requirements.
Structural Analysis
Determine whether modification groups are intact, providing a reliable basis for structural studies.
Functional Studies
Ensure the accuracy of drugs used in downstream functional experiments to avoid misinterpretation caused by molecular weight deviations.
Pharmacokinetics and Pharmacodynamics Research
Verify the molecular integrity of drugs related to in vivo and in vitro metabolism.
FAQ
Q1: How Accurate Is the DNA/RNA Drug Molecular Weight Confirmation Service?
A1: MtoZ Biolabs uses high-resolution mass spectrometry platforms (such as Orbitrap, Q-TOF, and MALDI-TOF) combined with deconvolution algorithms to achieve sub-Dalton molecular weight measurement accuracy. This not only precisely verifies the consistency between the target nucleic acid and the theoretical sequence but also detects truncated products, missing modifications, and low-abundance impurities, ensuring scientifically reliable results.
Q2: Is the Service Applicable to Different Types and Modifications of Nucleic Acid Drugs?
A2: Yes. Our molecular weight confirmation service is compatible with various nucleic acid types, including antisense oligonucleotides (ASOs), siRNA, mRNA, and aptamers. Whether involving common phosphorothioate linkages and methylation or more complex chemical modifications such as lipid conjugation and PEGylation, we have the analytical expertise and platforms to deliver reliable molecular weight confirmation results.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Total Ion Chromatogram & Quality Control Assessment (project-dependent)
4. Data Analysis, Preprocessing, and Estimation (project-dependent)
5. Bioinformatics Analysis
6. Raw Data Files
Related Services
DNA/RNA Drug Optical Rotation Testing Service
How to order?







