DNA/RNA Drug pH Testing Characterization Service
- Mechanism of Action Studies
- Preclinical Studies
- Clinical Trial Support
- Commercial Manufacturing
- Post-Marketing Surveillance
DNA/RNA Drug pH Testing Characterization Service is an analytical service designed for evaluating the physicochemical properties of oligonucleotide drugs, aiming to comprehensively assess their acid-base characteristics and the impact on stability, solubility, and functional activity through precise pH testing. The pH value is one of the core parameters in nucleic acid drug quality control, as it not only determines the conformation and binding capacity of the drug in both in vivo and in vitro environments but also directly influences storage conditions, formulation design, and the safety and efficacy of clinical applications.
During the research and production of nucleic acid drugs, different pH conditions may cause degradation of the nucleotide backbone, instability of chemical modification groups, or significant changes in solubility, which in turn affect the pharmacokinetic properties and therapeutic outcomes of the drug. Therefore, systematic pH testing not only helps researchers determine the optimal storage range and formulation conditions of the drug but also enables early identification of potential degradation pathways and stability risks, providing critical data support for preclinical development, process optimization, and quality control.
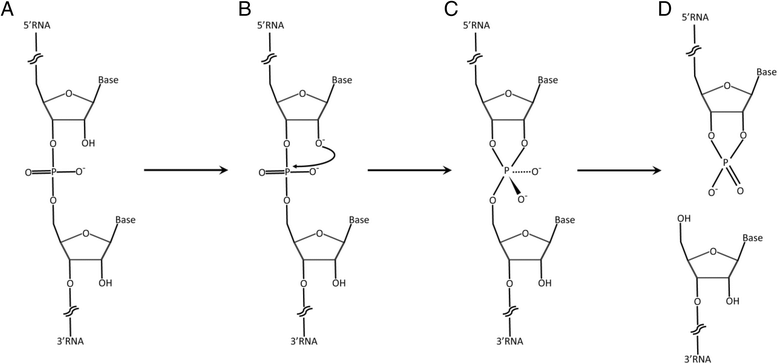
Lemire KA. et al. Virol J. 2016.
Figure 1. Schematic of RNA degradation under extreme alkaline pH conditions.
Services at MtoZ Biolabs
By integrating high-precision pH electrode testing with multidimensional analytical methods such as high-performance liquid chromatography (HPLC) and capillary electrophoresis (CE), MtoZ Biolabs provides DNA/RNA Drug pH Testing Characterization Service to comprehensively evaluate drug stability, solubility, and degradation behavior under different pH conditions, thereby delivering solid data support for research, quality control, and regulatory submission.
Our service scope covers several key areas:
pH Stability Evaluation: Systematically assess drug stability across different pH ranges to determine optimal storage and formulation conditions.
pH-Dependent Solubility Testing: Reveal the solubility profile of the drug and predict its behavior in both in vivo and in vitro environments.
Forced Degradation Studies: Simulate extreme conditions to identify potential degradation pathways and vulnerable sites, providing valuable insights for formulation design.
Comparative Stability Analysis: Compare the performance of different formulations or batches under various pH conditions to identify the most stable formulation and enhance batch-to-batch consistency.
Method Development and Validation: Establish and validate pH testing methods for nucleic acid drugs using platforms such as HPLC and CE to ensure accuracy and reproducibility of results.
Analysis Workflow
The general workflow of DNA/RNA Drug pH Testing Characterization Service is as follows:
1. Sample Preparation
Dissolve DNA/RNA drugs in appropriate buffers or solvents, and perform filtration or desalting if necessary to remove impurities.
2. pH Condition Setup
Expose samples to solutions with different pH ranges, covering both physiological and storage conditions.
3. Analytical Testing
Measure pH with high-precision electrodes and use techniques such as HPLC and CE to monitor drug concentration, purity, and degradation.
4. Data Collection and Processing
Record changes in pH, stability, and solubility under different conditions, and perform trend analysis using statistical methods.
5. Result Interpretation and Reporting
Provide a complete report including stability curves, solubility data, and comparative analysis.
Service Advantages
Advanced Analysis Platform: MtoZ Biolabs established an advanced DNA/RNA Drug pH Testing Characterization Service platform, guaranteeing reliable, fast, and highly accurate analysis service.
Comprehensive Methodological Support: Cover stability, degradation, solubility, and comparative studies to provide multidimensional data.
Rigorous Quality Control: Standardized operating procedures and multi-level quality control systems ensure accuracy and reproducibility of data.
End-to-End Technical Support: Deliver not only testing data but also result interpretation along with recommendations for research and regulatory submission.
Sample Submission Suggestions
Sample Types
Acceptable samples include DNA drugs, RNA drugs, and oligonucleotides (modified or unmodified).
Storage and Transportation
Store samples at low temperature (-20°C or below). Shipment on dry ice or under controlled temperature conditions is recommended.
It is recommended to contact the MtoZ Biolabs technical team prior to sample submission to obtain detailed and tailored guidelines for sample preparation and submission.
Applications
Reveal changes in solubility and activity of nucleic acid drugs under in vivo and in vitro conditions, providing evidence for mechanism research.
Evaluate drug stability under different pH conditions to optimize formulation design.
Verify drug quality consistency to ensure safety and efficacy.
Compare stability data across different batches to ensure consistency in large-scale production.
Continuously monitor the stability performance of drugs during storage and use.
FAQ
Q1: What Is the Scope of DNA/RNA Drug pH Testing Characterization Service?
A1: Our service not only provides precise pH measurement but also combines methods such as HPLC and CE to evaluate drug stability, solubility, and potential degradation products under different pH conditions.
Q2: How Is Accuracy and Reproducibility Ensured During pH Testing?
A2: MtoZ Biolabs uses high-precision pH electrodes with multipoint calibration, combined with a temperature compensation system to ensure stable measurement results. Standard buffer solutions are applied as controls during experiments, and cross-validation with HPLC and CE is performed to guarantee accuracy and reproducibility of the data.
Q3: What Does the Result Report Include?
A3: The report includes pH testing data under different conditions, stability and solubility curves, degradation and batch comparison analyses, as well as recommendations for storage conditions or formulation optimization.
Related Services
DNA/RNA Drug Molecular Weight Confirmation Service
How to order?







