Pharmaceutics Analysis Service
Pharmaceutics is an important discipline in pharmaceutical sciences that focuses on drug formulation, prescription design, quality control, and the study of drug behavior both in vitro and in vivo. It bridges pharmaceutical chemistry and clinical application, serving as a key process for transforming active pharmaceutical ingredients into safe, effective, and stable dosage forms. As modern drug development becomes increasingly complex, with the rise of novel formulations such as nanomedicines, liposomes, controlled-release systems, and nucleic acid drugs, the technical requirements for pharmaceutics analysis continue to increase.
Pharmaceutics analysis systematically evaluates drug purity, stability, structural consistency, and release characteristics through multidimensional physicochemical detection and data interpretation, providing reliable support for drug development, production optimization, and regulatory submission.
Equipped with advanced mass spectrometry, chromatography, spectroscopy, and thermal analysis platforms, MtoZ Biolabs has established a comprehensive Pharmaceutics Analysis Service system. This service covers multiple aspects ranging from microbial limit testing, impurity analysis, structure confirmation, composition testing, API analysis, intermediate analysis, excipient analysis, to nucleic acid drug characterization, providing high-quality and traceable analytical data for pharmaceutical companies, research institutions, and regulatory agencies.
Services at MtoZ Biolabs
MtoZ Biolabs integrates multiple high-resolution analytical technologies and standardized testing procedures. Our pharmaceutics analysis service includes:
Detailed Service Description
1. Drug Microbial Limit Testing Service
MtoZ Biolabs provides a professional Drug Microbial Limit Testing Service to help clients detect microbial contamination in raw materials, excipients, and finished pharmaceuticals products, and to verify compliance with regulatory requirements.
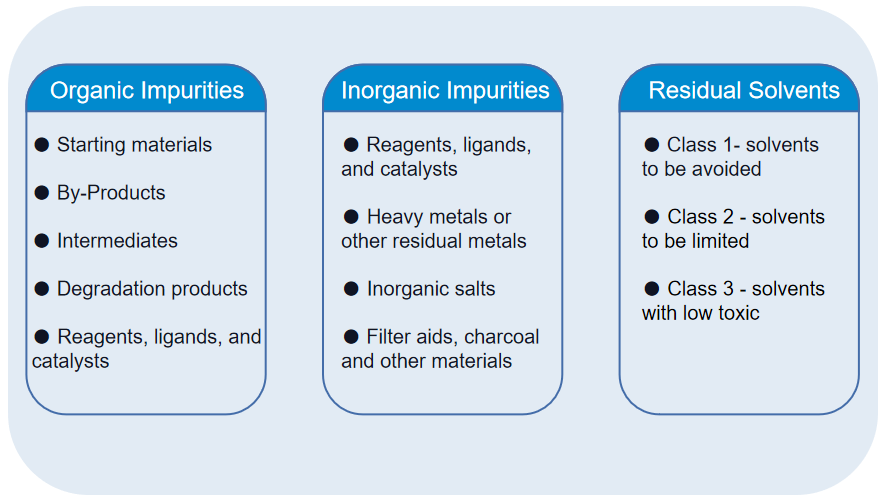
2. Pharmaceutical Impurity Analysis Service
MtoZ Biolabs offers comprehensive Pharmaceutical Impurity Analysis Service to support all stages of drug research, development, and manufacturing. Our laboratory platform integrates advanced chromatography, high-resolution mass spectrometry, and regulatory-compliant workflows to deliver accurate and reproducible impurity profiling.
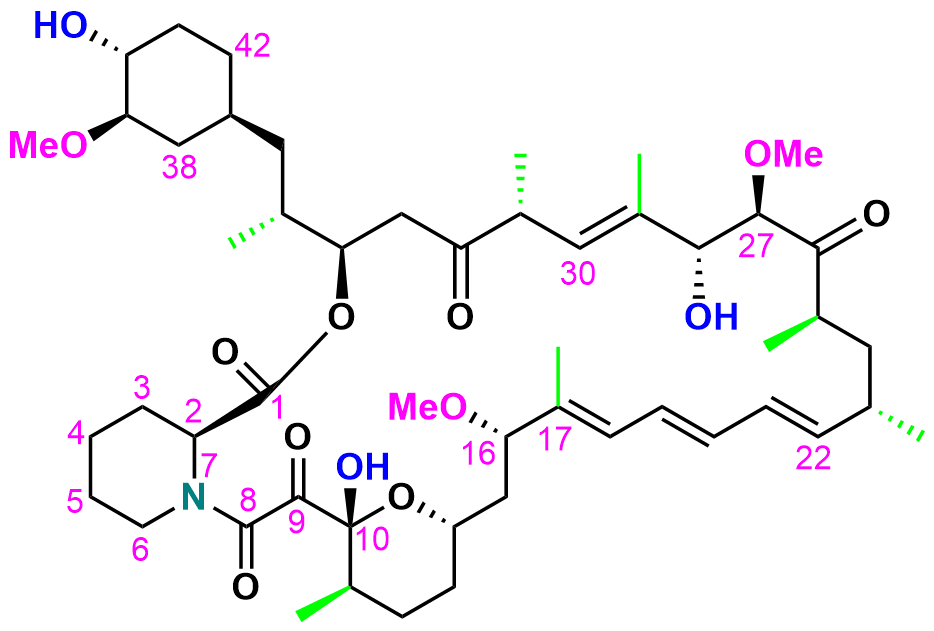
3. Drug Structure Confirmation Service
MtoZ Biolabs provides a comprehensive Drug Structure Confirmation Service that integrates advanced analytical methods to characterize atomic connectivity, stereochemistry, and molecular conformation.
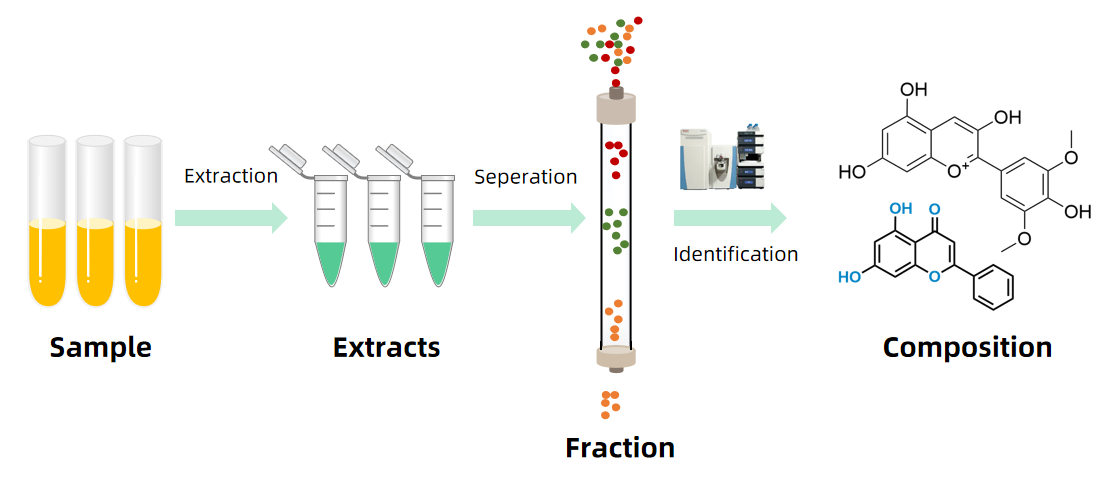
4. Drug Composition Testing Service
MtoZ Biolabs offers a comprehensive Drug Composition Testing Service that integrates state-of-the-art instrumentation with expert data interpretation. Our Drug Composition Testing Services help pharmaceutical companies ensure product quality, comply with international standards, and accelerate the path from research to commercialization.
5. API Analysis Service
MtoZ Biolabs offers specialized API Analysis Services to address these challenges. Our API Analysis Service portfolio combines advanced chromatographic, spectroscopic, and mass spectrometric platforms with validated methodologies to deliver accurate, reliable, and regulatory-compliant results. By partnering with us, clients benefit from end-to-end analytical solutions that support drug discovery, clinical development, manufacturing, and regulatory submissions.
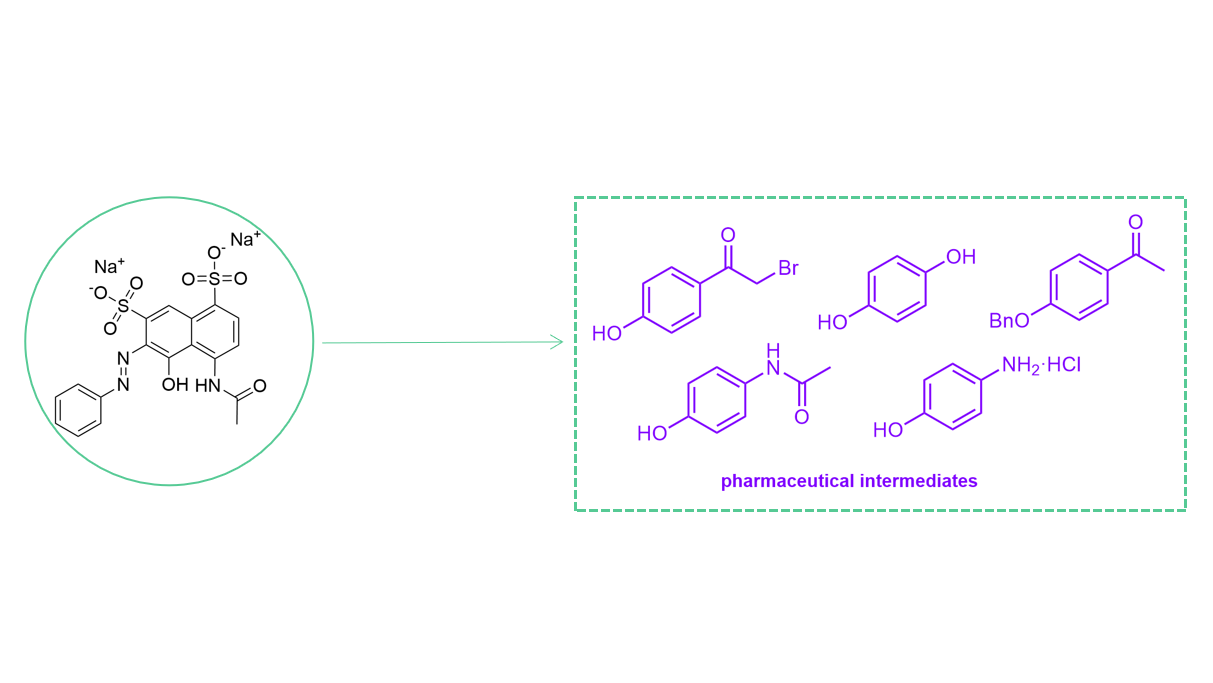
6. Pharmaceutical Intermediates Analysis Service
MtoZ Biolabs offers a comprehensive Pharmaceutical Intermediates Analysis Service designed to support drug discovery, development, and manufacturing. Our advanced analytical platforms, including mass spectrometry, nuclear magnetic resonance, chromatography, and spectroscopic methods, allow us to deliver reliable and regulatory-ready results. By combining technical expertise with customized workflows, we ensure that clients receive accurate data and actionable insights for every project.
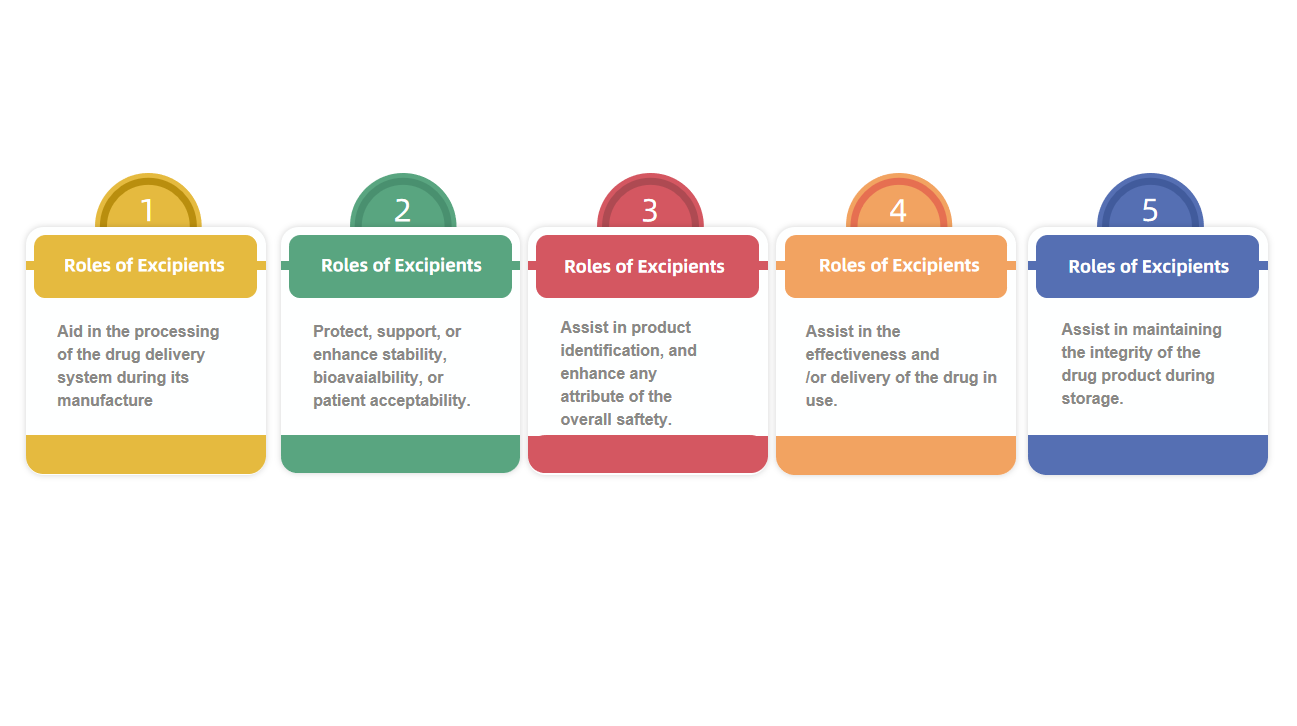
7. Pharmaceutical Excipient Analysis Service
MtoZ Biolabs provides comprehensive Pharmaceutical Excipient Analysis Service including purity testing, particle size distribution, thermal performance, hygroscopicity, and compatibility with active ingredients. Using FTIR, GC, HPLC, DSC, and SEM, we help clients select optimal excipient systems and support documentation for regulatory submission.
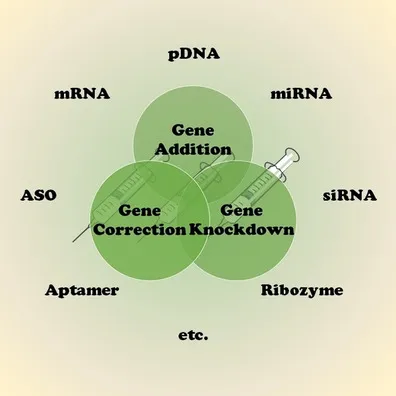
8. DNA/RNA Drug Characterization Service
DNA/RNA Drug Characterization Service refers to a professional service that provides systematic analysis and characterization of DNA and RNA drugs, aiming to evaluate their molecular structure, chemical properties, physical attributes, and biological activity. With the widespread clinical development of nucleic acid drugs such as gene therapies, mRNA vaccines, siRNA, and antisense oligonucleotides (ASOs), ensuring the purity, integrity, stability, and efficacy of these innovative therapies has become a critical aspect of research and production.
Service Advantages
1. Comprehensive Platforms
MtoZ Biolabs combines chromatography, mass spectrometry, spectroscopy, thermal analysis, and microscopic techniques to provide multidimensional testing from molecular structure to formulation performance. Our platform supports comprehensive research on solid, liquid, controlled-release, and nanomedicine dosage forms.
2. Quality Assurance
All experiments follow GLP and ISO quality management systems and strictly adhere to pharmacopeial and ICH standards, ensuring scientific accuracy, data integrity, and international credibility.
3. Professional Team
Our research team has expertise in pharmaceutics, chemistry, biology, and analytical sciences. With deep knowledge of regulatory requirements and quality studies, we provide clients with technical consultation and data interpretation support.
4. Customized Service
Whether in early formulation screening, process optimization, or post-marketing quality evaluation, MtoZ Biolabs delivers flexible and customized analytical solutions designed to balance accuracy and efficiency.
Applications
✔️ New drug formulation and dosage form development
✔️ Generic drug equivalence studies
✔️ Process optimization and manufacturing control
✔️ Drug stability and shelf-life evaluation
✔️ API and excipient quality assessment
✔️ Biologics and nucleic acid drug characterization
✔️ Regulatory submission and compliance support
Pharmaceutics analysis is the scientific foundation for ensuring drug quality, safety, and efficacy.
Contact our scientific team to make your drug development more accurate, efficient, and competitive.
How to order?