DNA/RNA Drug pKa Determination Service
DNA/RNA Drug pKa Determination Service is a specialized analytical service for measuring the acid dissociation constant of ionizable groups in nucleic acid drugs. The pKa value is an important parameter reflecting the acid-base properties of a molecule, as it determines the ionization state and charge distribution of the molecule under different pH conditions and directly influences the solubility, stability, membrane permeability, and bioavailability of nucleic acid drugs.
pKa measurement plays a crucial role in the development of DNA and RNA drugs. It can reveal how drugs behave in various acidic and alkaline environments in vivo and in vitro, providing a basis for dose planning, formulation development, and storage optimization. It is also a key indicator for pharmacokinetic studies, safety evaluation, and quality control. Without accurate knowledge of pKa, drugs may rapidly degrade in vivo, lose activity, or induce adverse reactions, hindering clinical translation and commercial production. Therefore, systematic pKa measurement for DNA/RNA drugs is not only essential for the basic characterization of nucleic acid drugs but also a crucial factor in promoting their efficient development and regulatory compliance.
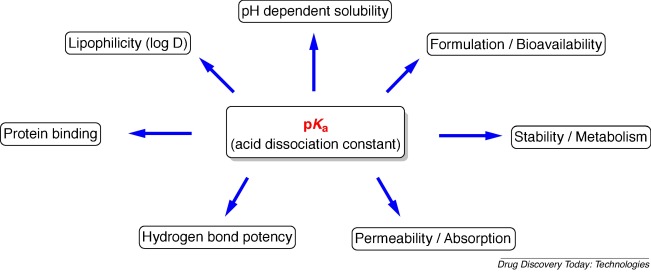
Dardonville C. Drug Discov Today Technol. 2018.
Figure 1. Impact of pKa on compounds physicochemical properties and interactions with other parameters.
Services at MtoZ Biolabs
MtoZ Biolabs provides DNA/RNA Drug pKa Determination Service to systematically analyze the dissociation behavior and physicochemical properties of nucleic acid drugs under different acidic and alkaline conditions. We employ multiple complementary techniques, including potentiometric titration, spectroscopic methods, and chromatographic methods, to ensure accurate, reproducible, and interpretable pKa data.
In addition to the core service of precise determination of pKa values for DNA and RNA drug candidates, MtoZ Biolabs also offer:
Method Development and Validation: Customize and validate experimental protocols based on drug characteristics to ensure precise and reproducible pKa testing.
Bioavailability Prediction: Use pKa data to predict drug solubility, absorption efficiency, and pharmacokinetic behavior under different physiological conditions.
Safety Evaluation: Combine ionization state analysis to identify potential safety risks in advance and provide risk-control references for drug development.
Stability Studies: Assess chemical stability of drugs in different pH environments and identify potential degradation pathways.
Analysis Workflow
The general workflow of DNA/RNA Drug pKa Determination Service:
1. Sample Preparation
Process DNA/RNA drug samples according to standardized requirements.
2. Experimental Measurement
Perform pKa determination and stability studies using potentiometric titration, spectroscopic methods, or HPLC/CE platforms.
3. Data Analysis
Conduct curve fitting and mathematical modeling of the experimental data to calculate the pKa values of ionizable groups in the drug.
4. Result Analysis and Interpretation
Analyze experimental results in relation to stability, solubility, and pharmacokinetics, and provide recommendations for formulation optimization or storage conditions.
5. Report Delivery
Provide a complete technical report including experimental conditions, measurement results, graphical data, and professional interpretation.
Service Advantages
Advanced Analysis Platform: MtoZ Biolabs established an advanced DNA/RNA Drug pKa Determination Service platform, guaranteeing reliable, fast, and highly accurate analysis service.
Standardized Quality Control: Apply standardized operating procedures and strict quality control systems to ensure scientific reliability of data.
Professional Research Team: Possess extensive experience in nucleic acid drug characterization and provide customized solutions.
Extended Data Value: Deliver not only pKa determination results but also comprehensive analysis combined with stability, solubility, and safety evaluation.
Sample Submission Suggestions
Sample Type
Applicable to a variety of samples, including DNA drugs, RNA drugs, and modified or unmodified oligonucleotides.
Storage and Transportation
Low-temperature storage (-20°C or below) is recommended. Dry ice or temperature-controlled packaging is recommended for transportation.
It is recommended to contact the MtoZ Biolabs technical team prior to sample submission to obtain detailed and tailored guidelines for sample preparation and submission.
Applications
Drug Development: Applied to the screening of physicochemical properties and structural optimization of early candidate molecules.
Pharmacokinetics and Bioavailability Studies: Use pKa data to predict drug behavior in vivo.
Safety and Toxicity Evaluation: Identify potential side effects or toxicity risks associated with ionization states.
Dose Optimization: Combine pKa and solubility data to provide scientific evidence for clinical dosing strategies.
FAQ
Q1: How Is the Accuracy and Reproducibility of pKa Data Ensured?
A1: MtoZ Biolabs uses multipoint calibration and standard buffer validation combined with an automatic temperature compensation system to ensure measurement precision. Experiments are typically repeated more than three times, and reference standards are used for comparison when necessary, thereby guaranteeing the accuracy and reproducibility of the data.
Q2: Can pKa Determination Be Performed on Modified Nucleic Acid Drugs?
A2: Yes. Our methods are compatible with various modification forms such as phosphorothioate, lipid conjugation, and PEGylation, and experimental conditions can be optimized to ensure scientific reliability and data comparability.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. pKa Determination Data
4. Stability and Solubility Analysis (project-dependent)
5. Comparative Results and Conclusions (project-dependent)
6. Raw Data Files
Related Services
DNA/RNA Drug Molecular Weight Confirmation Service
How to order?







