DNA/RNA Drug Quantitative Analysis Service
- Drug Development and Formulation Optimization
- Preclinical and Clinical Research
- Precision Medicine
- Biomarker Research
- Quality Control and Regulatory Submission
DNA/RNA Drug Quantitative Analysis Service refers to an analytical service that accurately measures the actual content and concentration of nucleic acid drugs using multiple approaches such as optical, chromatographic, and mass spectrometric methods. With high sensitivity and specificity in quantitative detection, clients can obtain reliable data required for drug dosage design, quality control, pharmacokinetic analysis, and regulatory submission.
With the rapid development of nucleic acid drugs, they have become an essential pillar of precision medicine and innovative therapies. The efficacy, safety, and batch-to-batch consistency of nucleic acid drugs largely depend on accurate quantitative analysis. By precisely determining drug content in formulations and biological systems, researchers can not only optimize dosage design but also effectively monitor stability and distribution in vivo. Therefore, DNA/RNA drug quantitative analysis is not only the foundation for formulation optimization and pharmacokinetic studies but also an indispensable step in regulatory submission, clinical research, and commercial production, providing solid data support for the development and application of nucleic acid drugs.
Services at MtoZ Biolabs
MtoZ Biolabs provides DNA/RNA Drug Quantitative Analysis Service that not only evaluates the dosage and purity of nucleic acid drugs in formulations but also measures their distribution and stability in complex biological matrices such as plasma, tissues, and cells. Our analytical service integrates molecular biology, chromatography, and mass spectrometry approaches to deliver highly sensitive, specific, and reproducible quantification solutions for nucleic acid drugs.
qPCR/dPCR: Based on fluorescence signal amplification, these methods enable precise quantification of nucleic acid drugs. Digital PCR (dPCR) achieves absolute quantification and ultra-high sensitivity through reaction partitioning, making it especially suitable for detecting low-abundance molecules.
Ligation-Dependent qPCR: Enhances detection sensitivity and specificity through sequence-specific ligation reactions, particularly useful for detecting low-abundance nucleic acids in complex biological matrices.
Hybridization-Fluorescence Detection: Employs fluorescence-labeled specific probes combined with liquid chromatography separation to achieve highly specific and high-resolution quantification of oligonucleotides.
LC-MS/MS and LC-HRAM: Utilizes liquid chromatography coupled with tandem mass spectrometry or high-resolution accurate mass spectrometry to perform quantitative analysis of nucleic acid drugs, especially suitable for modified nucleic acids and simultaneous multi-target analysis.
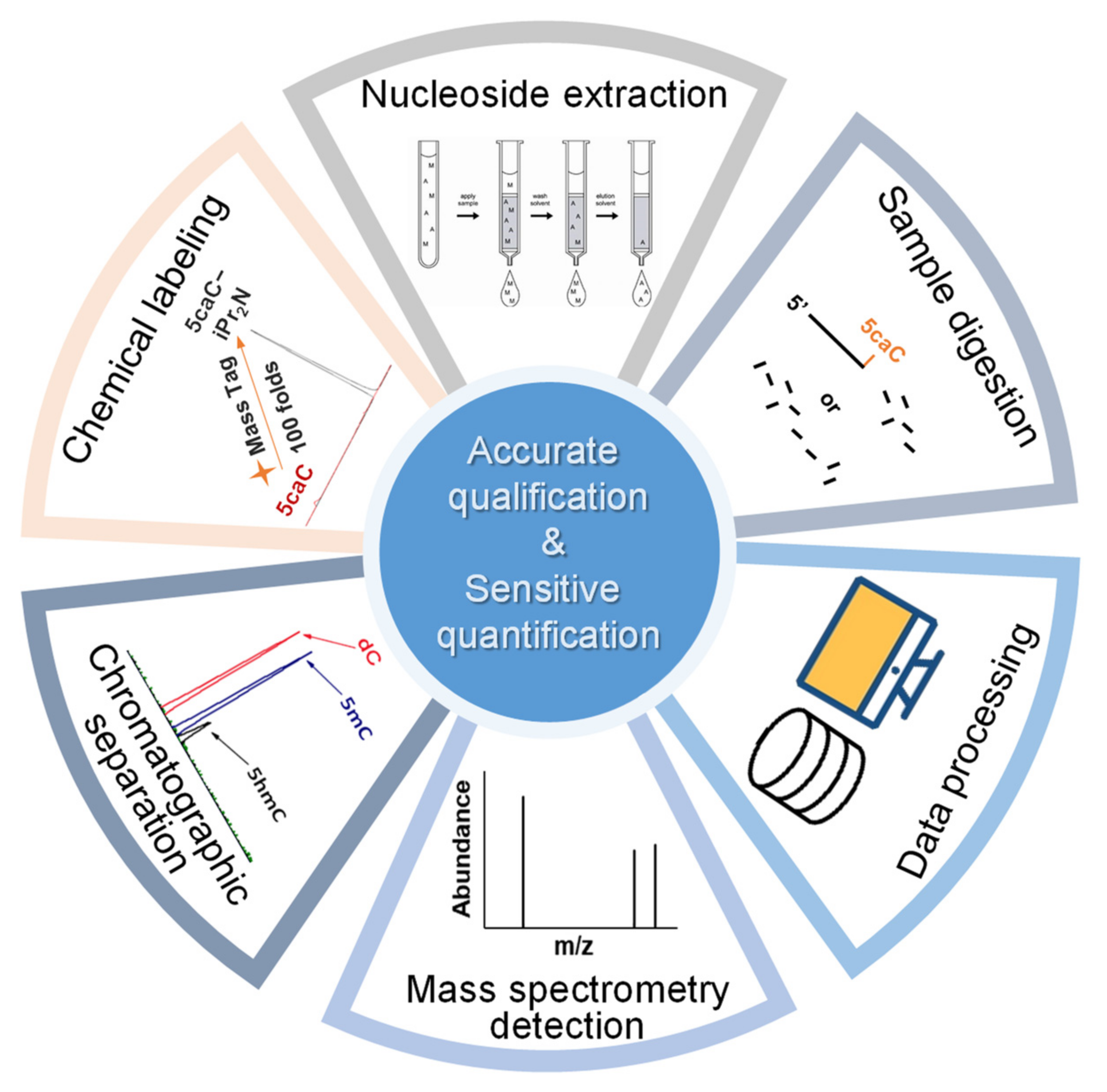
Liu, Y. et al. Int J Mol Sci. 2024.
Figure 1. Process of mass spectrometry based method for accurate qualification and sensitive quantification of nucleic acid modification.
Analysis Workflow
The general workflow of DNA/RNA Drug Quantitative Analysis Service is as follows:
1. Sample Preparation
Extract and purify DNA/RNA from samples to ensure detection sensitivity and accuracy.
2. Method Selection and Optimization
Select and optimize the detection method based on nucleic acid type and research objectives, and design specific primers/probes.
3. Quantitative Detection
Perform analysis under standardized conditions to obtain concentration data, using multiple platforms for cross-validation of accuracy when necessary.
4. Data Analysis
Analyze the acquired data using advanced algorithms and software tools.
5. Result Report
Provide a complete report including quantitative data, experimental conditions, raw spectra, and result interpretation.
Service Advantages
Advanced Analysis Platform: MtoZ Biolabs established an advanced DNA/RNA Drug Quantitative Analysis Service platform, guaranteeing reliable, fast, and highly accurate analysis service.
One-Time-Charge: Our pricing is transparent, no hidden fees or additional costs.
High-Data-Quality: Deep data coverage with strict data quality control. AI-powered bioinformatics platform integrates all DNA/RNA Drug Quantitative Analysis data, providing clients with a comprehensive data report.
Customized Analytical Solutions: Provide personalized analytical strategies based on different drug types and development stages.
Sample Submission Suggestions
Sample Types
Acceptable samples include purified DNA drugs, RNA drugs, plasma, cells, and other sample types.
Storage and Transportation
Recommended storage at low temperature (-20°C or below), with shipment on dry ice or under temperature-controlled conditions.
It is recommended to contact the MtoZ Biolabs technical team prior to sample submission to obtain detailed and tailored guidelines for sample preparation and submission.
Applications
Provide quantitative data support for dose design, stability studies, and formulation development.
Assess the distribution, stability, and pharmacokinetic characteristics of nucleic acid drugs in vivo.
Monitor nucleic acid drug levels in patients to enable personalized dose adjustments.
Support the application of novel nucleic acid biomarkers in disease diagnosis and therapeutic monitoring.
Provide compliance testing data for batch-to-batch consistency evaluation and regulatory filing.
FAQ
Q1: What Are the Differences in Applicability Among Different Platforms for DNA/RNA Drug Quantitative Analysis Service?
A1: qPCR is suitable for routine quantification with high sensitivity but depends on standard curves; dPCR provides absolute quantification with superior reproducibility and sensitivity, particularly suitable for low-abundance nucleic acids; LC-MS/MS and LC-HRAM are especially applicable for modified nucleic acids and complex samples, allowing simultaneous detection of multiple nucleic acids and metabolites; hybridization-fluorescence assays have distinct advantages in the specific quantification of oligonucleotides.
Q2: Which Methods Are Most Suitable for Quantification of Modified Nucleic Acid Drugs?
A2: LC-MS/MS and LC-HRAM are the preferred methods for quantifying modified nucleic acids, as they can accurately distinguish intact molecules, modified forms, and degradation products. Additionally, we can combine dPCR or hybridization-fluorescence assays for cross-validation to ensure reliable results.
Related Services
DNA/RNA Drug Size and Aggregation Analysis Service
DNA/RNA Drug Quantification and Potency Determination Service
How to order?







