DNA/RNA Drug Quantification and Potency Determination Service
- Drug Development and Dose Optimization
- Formulation Development and Quality Control
- Preclinical and Clinical Research
- Scientific Research
- Regulatory Compliance Testing
DNA/RNA Drug Quantification and Potency Determination Service is a comprehensive analytical service designed to measure the content and evaluate the functional activity of nucleic acid drugs. Quantitative analysis aims to accurately determine the actual concentration and dosage of the drug to ensure consistency during research and production, while potency determination uses cellular or molecular functional assays to assess whether the drug exerts its intended biological effects, thereby confirming its therapeutic potential.
With the expanding applications of nucleic acid drugs such as antisense oligonucleotides (ASOs), small interfering RNA (siRNA), messenger RNA (mRNA), and aptamers in the therapeutic field, precise quantification and potency characterization have become central requirements for drug development and quality control. Quantitative analysis ensures accurate dosing and batch-to-batch consistency, while potency determination directly reflects the functional integrity and biological activity of the drug, serving as a critical step in validating the outcomes of nucleic acid drug development and their clinical translation value.
Services at MtoZ Biolabs
Relying on advanced analytical platforms and a comprehensive quality control system, MtoZ Biolabs provides DNA/RNA Drug Quantification and Potency Determination Service to perform precise quantification and biological potency evaluation of nucleic acid drugs. Our team of experts can customize testing strategies according to different drug types and development stages, ensuring sensitivity, accuracy, and reproducibility of results, and providing high-quality data support for clients in drug development, formulation optimization, quality control, and regulatory submission.
Analysis Workflow
The general workflow of DNA/RNA Drug Quantification and Potency Determination Service is as follows:
1. Sample Preparation
Receive and process DNA/RNA drug samples as required to ensure controlled testing conditions.
2. Analytical Method Development and Optimization
Establish or optimize quantification and potency testing methods based on drug characteristics to ensure sensitivity and reproducibility.
3. Quantitative Detection
Use methods such as HPLC, LC-MS, or capillary electrophoresis combined with standard curves and reference materials to obtain accurate drug concentration data.
4. Potency Evaluation
Conduct cell-based assays or in vitro functional tests to assess drug activity levels and compare results with standard controls.
5. Data Analysis
Generate dose-response curves and calculate potency parameters.
6. Report Generation
Provide a complete technical report including experimental conditions, raw data, statistical results, and scientific interpretation.
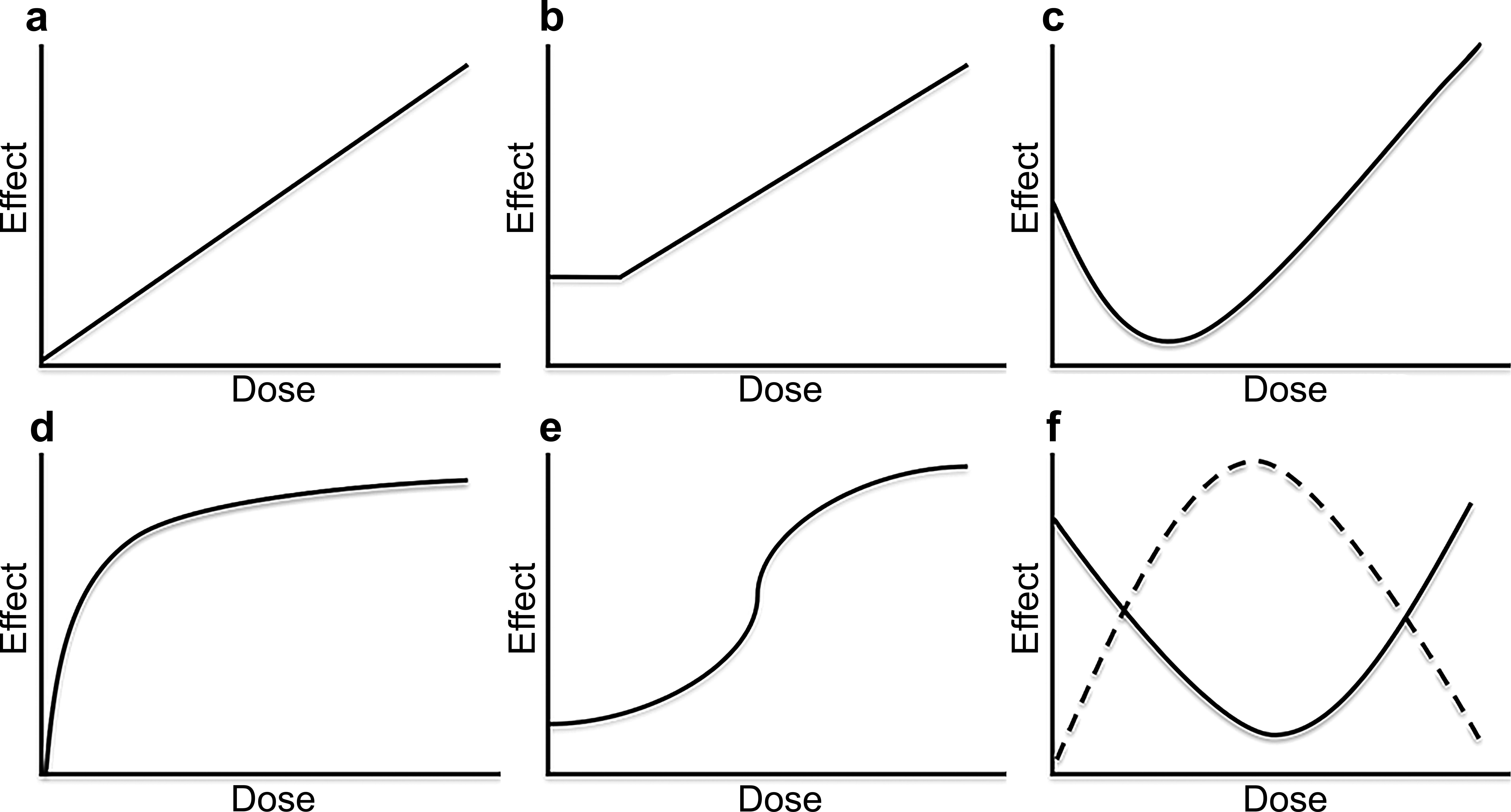
Ramaiah L. et al. oxicol Pathol. 2017.
Figure 1. Illustration of common dose-response curves.
Service Advantages
Advanced Analysis Platform: MtoZ Biolabs established an advanced DNA/RNA Drug Quantification and Potency Determination Service platform, guaranteeing reliable, fast, and highly accurate analysis service.
One-Time-Charge: Our pricing is transparent, no hidden fees or additional costs.
High-Data-Quality: Deep data coverage with strict data quality control. AI-powered bioinformatics platform integrates all DNA/RNA Drug Quantification and Potency Determination data, providing clients with a comprehensive data report.
Customized Solutions: Develop personalized testing workflows based on drug type and research objectives.
Sample Submission Suggestions
Sample Types
Accepts various forms of nucleic acid drugs, including DNA, RNA, siRNA, antisense oligonucleotides (ASOs), mRNA, aptamers, and their modified forms.
Storage and Transportation
Recommended storage at low temperature (-20°C or below) with shipment on dry ice or under temperature-controlled protection.
It is recommended to contact the MtoZ Biolabs technical team prior to sample submission to obtain detailed and tailored guidelines for sample preparation and submission.
Applications
Ensure accurate dosing of nucleic acid drugs to avoid insufficient efficacy or potential toxicity.
Used for batch-to-batch consistency evaluation and long-term stability studies.
Help confirm the biological function and pharmacokinetic characteristics of drugs, providing key data support for regulatory submission and clinical translation.
Applied in basic studies and investigations of novel nucleic acid molecular mechanisms.
Serve as an important basis for drug registration and regulatory review.
FAQ
Q1: What Strategies Are Used for Potency Determination in DNA/RNA Drug Quantification and Potency Determination Service?
A1: Potency determination can be performed at the cellular level, such as gene expression inhibition assays or reporter gene systems, or at the molecular level, such as enzymatic activity assays. Results are typically presented as dose-response curves, and parameters such as EC₅₀ or IC₅₀ are calculated to directly reflect the functional potency of the drug.
Q2: Can Modified Nucleic Acid Drugs (Such as Phosphorothioate, PEG Conjugation, or Lipid Modification) Be Quantified and Evaluated for Potency?
A2: Yes. MtoZ Biolabs applies LC-MS/MS along with customized method development to distinguish modified forms, intact molecules, and degradation products. Functional assay systems adapted to the modifications are then used to evaluate their impact on drug potency, ensuring specificity and accuracy of the results.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Total Ion Chromatogram & Quality Control Assessment (project-dependent)
4. Data Analysis, Preprocessing, and Estimation (project-dependent)
5. Raw Data Files
Related Services
DNA/RNA Drug Molar Extinction Coefficient Determination Service
How to order?







