DNA/RNA Drug Molar Extinction Coefficient Determination Service
- Nucleic Acid Drug Quantitative Analysis
- Purity Evaluation
- Drug Synthesis Quality Control
- Structural and Conformational Studies
- Biophysical and Pharmacokinetic Studies
DNA/RNA Drug Molar Extinction Coefficient Determination Service is a specialized analytical service that measures the light absorption capacity of nucleic acid drugs at specific wavelengths through optical analysis methods to obtain the molar extinction coefficient (ε). The molar extinction coefficient is a key parameter reflecting the UV absorption characteristics of DNA and RNA molecules, establishing a quantitative relationship between absorbance and concentration, and serving as the basis for nucleic acid drug concentration calculation, purity evaluation, and quality control.
In the research and production of nucleic acid drugs, accurate concentration information is fundamental for dose design, efficacy evaluation, and clinical safety. Although theoretical ε values can be estimated based on nucleotide sequences, the actual values are often influenced by nucleotide composition, secondary structure, solvent environment, and temperature, which leads to discrepancies between theoretical and experimental results. Therefore, experimental determination of the molar extinction coefficient has become an indispensable step in nucleic acid drug quality characterization.
By precisely determining the molar extinction coefficient, researchers can establish more reliable quantitative models to ensure that nucleic acid drugs meet strict quality standards during research, formulation optimization, and regulatory submission, thereby providing strong data support for clinical translation and commercial application.
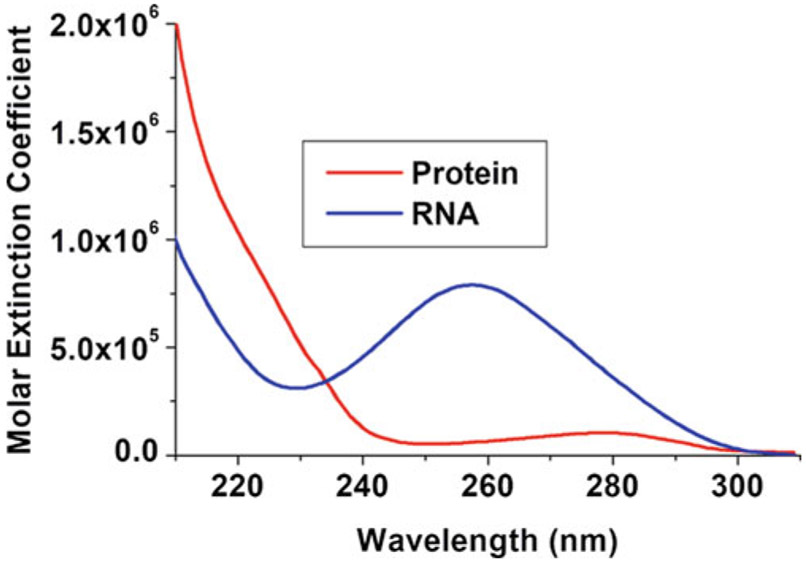
Mitra S. Demeler B. Methods Mol Biol. 2020.
Figure 1. Estimating RNA and protein absorbance for wavelength selection.
Services at MtoZ Biolabs
MtoZ Biolabs provides DNA/RNA Drug Molar Extinction Coefficient Determination Service to help obtain reliable molar extinction coefficient data. Using high-sensitivity spectrophotometers and other analytical techniques, we achieve precise quantification of different types of nucleic acid drugs, including modified oligonucleotides, siRNA, mRNA, and aptamers, through wavelength scanning, absorption peak optimization, and linear fitting. Our service delivers not only ε values but also experimental conditions, standard curves, and result interpretation, providing strong data support for clients in nucleic acid drug research, formulation optimization, and regulatory submission.
Analysis Workflow
The general workflow of DNA/RNA Drug Molar Extinction Coefficient Determination Service is as follows:
1. Sample Preparation
Purify DNA/RNA drug samples to remove impurities or contaminants that may interfere with absorbance measurement.
2. Concentration Series Preparation
Prepare a series of standard solutions with known concentrations to construct the absorbance–concentration calibration curve.
3. Spectral Measurement
Use a spectrophotometer to scan the absorbance of samples at different wavelengths and determine the maximum absorption peak.
4. Data Analysis
Calculate the molar extinction coefficient based on the Beer–Lambert law and validate it with the calibration curve.
5. Report Generation
Provide a complete technical report including the molar extinction coefficient value, experimental conditions, absorption spectra, and result interpretation.
Service Advantages
Advanced Analysis Platform: MtoZ Biolabs established an advanced DNA/RNA Drug Molar Extinction Coefficient Determination Service platform, guaranteeing reliable, fast, and highly accurate analysis service.
One-Time-Charge: Our pricing is transparent, no hidden fees or additional costs.
High-Data-Quality: Deep data coverage with strict data quality control. AI-powered bioinformatics platform integrates all DNA/RNA Drug Molar Extinction Coefficient Determination data, providing clients with a comprehensive data report.
Sample Submission Suggestions
Sample Types
Applicable to DNA drugs, RNA drugs, oligonucleotides (modified or unmodified), mRNA, and aptamers, etc.
Storage and Transportation
Store at low temperature (-20°C or below). Shipment on dry ice is recommended.
It is recommended to contact the MtoZ Biolabs technical team prior to sample submission to obtain detailed and tailored guidelines for sample preparation and submission.
Applications
Achieve precise quantification through ε values to ensure scientific and rational dose design.
Compare theoretical and experimental ε values to help determine the presence of impurities in samples.
Monitor quality and evaluate batch consistency during the production of nucleic acid drugs.
Investigate secondary structures and conformational changes based on absorption characteristics.
Provide data support for biophysical parameter analysis and pharmacokinetic modeling of drugs.
FAQ
Q1: What Methods Are Primarily Used in DNA/RNA Drug Molar Extinction Coefficient Determination Service?
A1: The most commonly used method is spectrophotometry, which is suitable for most DNA/RNA drug samples. For samples with high impurity levels or complex matrices, MtoZ Biolabs can also apply HPLC-UV separation followed by measurement to improve the accuracy and reliability of the data.
Q2: Why Might the Measured ε Value Differ from the Theoretical Value?
A2: Theoretical ε values are calculated based on an additive model of mononucleotide components and do not account for factors such as secondary structure, solvent environment, or modification groups. Measured values better reflect the actual optical properties of the sample and should therefore be considered the standard for quantitative analysis and quality control.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Molar Extinction Coefficient Value
4. Spectral Data
5. Data Analysis and Result Interpretation
6. Raw Data Files
Related Services
DNA/RNA Drug pH Testing Characterization Service
How to order?







