DNA/RNA Drug Moisture Content Determination Service
DNA/RNA Drug Moisture Content Determination Service is a specialized analytical service that quantitatively measures the water content in nucleic acid drug samples through multiple analytical techniques. Its primary goal is to accurately assess the ratio of free water to bound water in order to reflect the physicochemical stability of the drug during production, storage, and application.
In the research and production of DNA and RNA drugs, moisture content is one of the key factors determining drug stability and quality consistency. Excessive moisture can accelerate the hydrolysis of the nucleotide backbone or chemical modification groups, leading to drug degradation, reduced shelf life, and decreased efficacy, while insufficient moisture may disrupt the structure of lyophilized powders, affecting reconstitution efficiency and bioactivity. Therefore, precise determination of moisture content is not only an essential step to ensure the safety and effectiveness of nucleic acid drugs but also a core quality control parameter for formulation development, process optimization, long-term storage, and regulatory compliance.
Services at MtoZ Biolabs
MtoZ Biolabs provides DNA/RNA Drug Moisture Content Determination Service to comprehensively evaluate the moisture levels of nucleic acid drugs and offer data support for clients during drug development, formulation design, and commercial production. By combining multiple complementary analytical methods, we flexibly select the most appropriate approach based on sample characteristics and research needs to ensure accuracy, sensitivity, and reproducibility of results.
Karl Fischer Titration (KF)
A classical high-precision method for moisture determination, suitable for trace water detection with sensitivity reaching the ppm level.
Thermogravimetric Analysis (TGA)
Measures weight changes of the sample under heating to differentiate between free water and bound water, while also providing thermal stability evaluation.
Infrared Spectroscopy (IR)
Rapid quantification based on water absorption features at specific wavelengths, suitable for high-throughput screening and quality monitoring.
Gas Chromatography (GC)
Releases water under heating conditions followed by chromatographic detection, suitable for simultaneously monitoring water content and other volatile impurities.
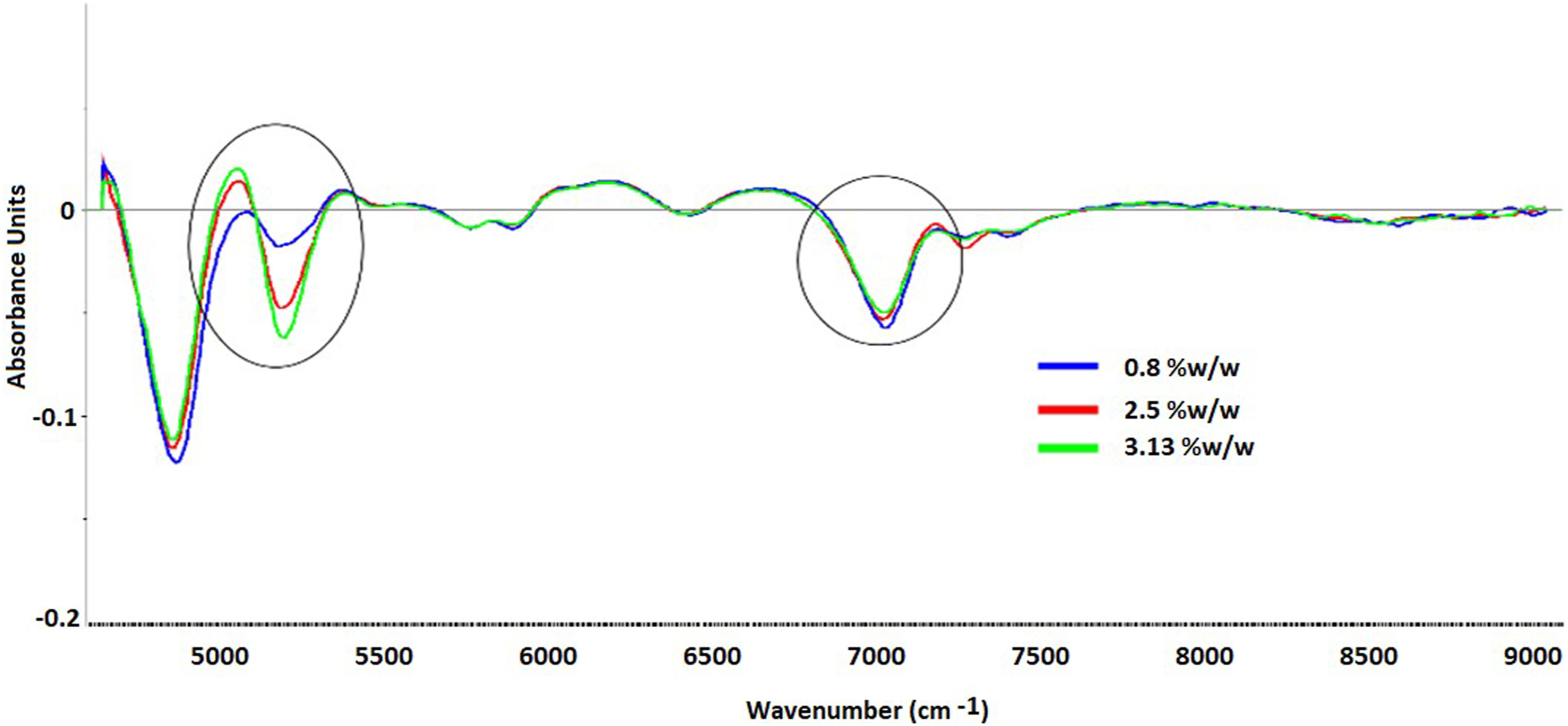
Khanolkar A. et al. J Near Infrared Spec. 2024.
Figure 1. Gradient change in NIR absorbance with the change in moisture content.
Analysis Workflow
The general workflow of DNA/RNA Drug Moisture Content Determination Service is as follows:
1. Sample Preparation
Perform necessary pretreatment of DNA/RNA drug samples.
2. Method Selection
Choose the appropriate analytical method based on sample properties and the expected moisture range.
3. Accurate Quantification
Conduct measurements following standardized procedures to ensure high sensitivity and reproducibility.
4. Data Analysis
Process raw data through calculation and statistical analysis to generate moisture content results and trend curves.
5. Report Generation
Provide a complete report including result data, analytical interpretation, and quality evaluation.
Service Advantages
Advanced Analysis Platform: MtoZ Biolabs established an advanced DNA/RNA Drug Moisture Content Determination Service platform, guaranteeing reliable, fast, and highly accurate analysis service.
Standardized Quality Control System: Strict experimental protocols and quality management procedures ensure reproducible results.
Extensive Drug Analysis Experience: The team is familiar with the characteristics of various DNA/RNA drugs and provides customized analytical solutions.
Extended Data Value: Deliver not only moisture content values but also formulation optimization and storage recommendations based on stability and quality control requirements.
Sample Submission Suggestions
Sample Types
Acceptable samples include DNA drugs, RNA drugs, modified or unmodified oligonucleotides, and aptamers, in either lyophilized powder or solution form.
Storage and Transportation
Lyophilized samples should be stored in a low-temperature dry environment, while solution samples should be stored at -20°C or below. Shipment on dry ice or under temperature-controlled conditions is recommended.
It is recommended to contact the MtoZ Biolabs technical team prior to sample submission to obtain detailed and tailored guidelines for sample preparation and submission.
Applications
Stability Studies: Evaluate the impact of moisture changes during storage and transportation on drug stability.
Batch Consistency Monitoring: Ensure quality comparability across different production batches.
Drug Development and Formulation Optimization: Guide the design of lyophilization processes and storage conditions to extend drug shelf life.
Dose Optimization: Ensure optimal reconstitution performance and efficacy at the time of administration.
FAQ
Q1: What Methods Are Commonly Used in DNA/RNA Drug Moisture Content Determination Service?
A1: MtoZ Biolabs offers multiple analytical platforms, including Karl Fischer titration (KF), thermogravimetric analysis (TGA), infrared spectroscopy (IR), and gas chromatography (GC). Each method has its advantages: KF enables trace detection at the ppm level, TGA distinguishes between bound and free water, IR is suitable for rapid screening, and GC allows simultaneous monitoring of moisture and volatile impurities. You only need to provide your requirements, and we will select the most appropriate method for your samples.
Q2: How Is Accuracy and Reliability of the Results Ensured?
A2: All experiments are conducted in temperature- and humidity-controlled environments and calibrated with standards and buffer solutions. Each sample is analyzed in at least three parallel experiments, and standard deviations are provided to ensure reproducibility and reliability of the results. You can confidently use the data for research and quality control.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Quantitative Moisture Content Results
4. Trend and Comparative Analysis
5. Stability and Quality Interpretation
6. Raw Data Files
Related Services
DNA/RNA Drug pH Testing Characterization Service
DNA/RNA Drug pKa Determination Service
DNA/RNA Drug Molar Extinction Coefficient Determination Service
How to order?







