DNA/RNA Drug Size and Aggregation Analysis Service
- Quality Control and Characterization
- Formulation Development
- Safety Evaluation
- Regulatory Testing
- Drug Delivery and Nanomedicine
DNA/RNA Drug Size and Aggregation Analysis Service is a specialized analytical service for evaluating the particle size distribution and aggregation state of nucleic acid drugs. By precisely measuring the hydrodynamic diameter, dispersity, and degree of aggregation of nucleic acid drugs under different conditions, researchers can gain a comprehensive understanding of their physical properties, which supports drug design, formulation optimization, and quality control.
In the research and production of nucleic acid drugs, particle size and aggregation are critical physicochemical parameters that directly affect drug stability, delivery efficiency, and pharmacokinetics in vivo. An appropriate particle size enhances cellular uptake efficiency and tissue distribution, while aggregation may lead to reduced activity, increased immunogenicity, or even adverse reactions. Therefore, size and aggregation analysis of DNA/RNA drugs is not only a key step in quality control and formulation development but also an essential requirement for regulatory submission and clinical application.
Services at MtoZ Biolabs
MtoZ Biolabs provides DNA/RNA Drug Size and Aggregation Analysis Service covering the full range of requirements from routine particle size measurement to complex aggregate characterization. By applying multiple complementary methods, including dynamic light scattering (DLS), size-exclusion chromatography (SEC), analytical ultracentrifugation (AUC), and transmission electron microscopy (TEM), we systematically characterize particle size distribution, monodispersity, and aggregation state of nucleic acid drugs. With strict quality control procedures, we ensure high precision and reproducibility of results, enabling clients to gain a comprehensive understanding of the structural features and physicochemical behavior of nucleic acid drugs and providing a solid data foundation for drug development and application.
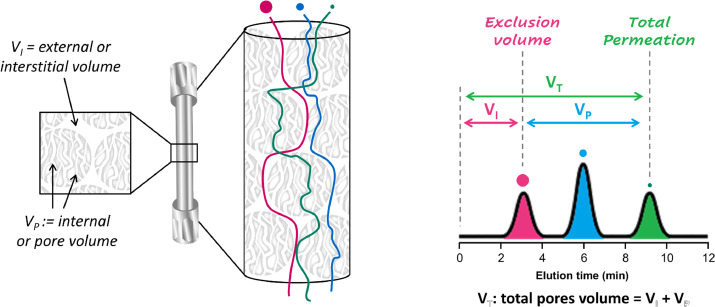
D'Atri V. et al. J Chromatogr A. 2024.
Figure 1. SEC principle of operation.
Analysis Workflow
The general workflow of DNA/RNA Drug Size and Aggregation Analysis Service is as follows:
1. Sample Preparation
Receive and process samples, adjusting buffer conditions and concentration as required for the experiment.
2. Method Selection
Choose the appropriate analytical platform based on sample characteristics and research objectives, such as DLS, SEC, microscopy, or electrophoresis.
3. Experimental Measurement
Perform testing under strict temperature control and quality management conditions, recording all data.
4. Data Analysis
Use specialized software and algorithms to analyze particle size distribution, PDI (polydispersity index), aggregation ratio, and morphological features.
5. Report Generation
Provide a complete technical report including raw data, spectra, experimental conditions, and result interpretation.
Service Advantages
Advanced Analysis Platform: MtoZ Biolabs established an advanced DNA/RNA Drug Size and Aggregation Analysis Service platform, guaranteeing reliable, fast, and highly accurate analysis service.
One-Time-Charge: Our pricing is transparent, no hidden fees or additional costs.
High-Data-Quality: Deep data coverage with strict data quality control. AI-powered bioinformatics platform integrates all DNA/RNA Drug Size and Aggregation Analysis data, providing clients with a comprehensive data report.
High Precision and Reliability: Strict quality control systems and multiple parallel experiments ensure data reproducibility and credibility.
Broad Sample Compatibility: Applicable to various types of nucleic acid drugs, including siRNA, ASOs, mRNA, aptamers, and modified oligonucleotides.
Sample Submission Suggestions
Sample Types
Supported samples include DNA, RNA, siRNA, antisense oligonucleotides, mRNA, aptamers, and their modified forms.
Storage and Transportation
Recommended storage at low temperature (-20°C or below), with shipment on dry ice or under temperature-controlled conditions.
It is recommended to contact the MtoZ Biolabs technical team prior to sample submission to obtain detailed and tailored guidelines for sample preparation and submission.
Applications
Evaluate particle size consistency and aggregation state of nucleic acid drugs across different batches for production process monitoring.
Assess the impact of different buffer systems, excipients, and processing conditions on particle size and aggregation.
Aggregation levels are associated with immunogenicity and toxicity, making them important for risk control.
Serve as key physicochemical indicators of nucleic acid drug stability and consistency to support compliance testing.
Provide fundamental data for carrier design and optimization of nucleic acid drug delivery systems.
FAQ
Q1: What Analytical Methods Are Commonly Used in DNA/RNA Drug Size and Aggregation Analysis Service?
A1: Common methods include dynamic light scattering (DLS), which is suitable for rapid measurement of particle size distribution and polydispersity; SEC, which quantitatively distinguishes monomers from aggregates; analytical ultracentrifugation (AUC), which provides precise quantification for complex samples; and TEM imaging, which directly visualizes particle morphology. MtoZ Biolabs typically selects single or combined methods based on sample characteristics and research objectives.
Q2: Can Testing Conditions Simulate In Vivo Environments?
A2: Yes. We can adjust pH, ion concentration, and temperature according to client requirements to simulate different physiological conditions or application environments, thereby generating data with greater practical relevance.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Particle Size Distribution Data
4. Aggregation Analysis Results
5. Experimental Spectra and Imaging
6. Raw Data Files
Related Services
DNA/RNA Drug Molar Extinction Coefficient Determination Service
DNA/RNA Drug Melting Temperature (Tm) Testing Service
DNA/RNA Drug Quantification and Potency Determination Service
How to order?







