DNA/RNA Drug Melting Temperature (Tm) Testing Service
- PCR Primer Design and Optimization
- Oligonucleotide Hybridization Experiments
- siRNA and RNAi Research
- Antisense Oligonucleotide Drug Development
- Gene Editing Applications
- Formulation Development and Quality Control
DNA/RNA Drug Melting Temperature (Tm) Testing Service is a specialized analytical service that measures the characteristic temperature (Tm value) at which nucleic acid drugs transition from double-stranded or ordered secondary structures to single-stranded or disordered states under controlled heating conditions. This parameter directly reflects the thermal stability and structural strength of nucleic acid molecules and is an indispensable physicochemical indicator in nucleic acid drug research, formulation optimization, and quality control.
In the development of nucleic acid drugs, the Tm value is a key factor for evaluating drug stability and functional reliability in both in vivo and in vitro environments. The value is influenced not only by nucleotide sequence, strand length, and GC content but also by external conditions such as ion concentration, pH, and buffer systems. Through precise quantification of Tm, researchers can improve primer design, optimize oligonucleotide hybridization, select antisense drug candidates, validate siRNA functionality, and design or refine guide RNAs, while also providing reliable quality control data for clinical development.
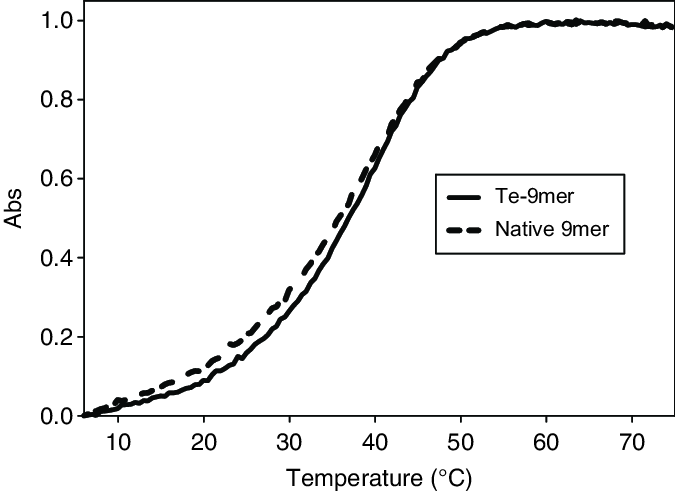
Sheng J. et al. Nucleic Acids Res. 2011.
Figure 1. Normalized thermal-denaturation curves of the native and Te-modified DNA duplexes.
Services at MtoZ Biolabs
MtoZ Biolabs provides DNA/RNA Drug Melting Temperature (Tm) Testing Service to perform precise thermal stability measurements for different types of nucleic acid drugs. By integrating spectrophotometry, differential scanning calorimetry (DSC), and circular dichroism (CD), we deliver highly sensitive and reproducible Tm testing data under strict temperature control and standardized quality management systems. Each report includes professional interpretation to help clients optimize molecular design, verify drug quality, and support regulatory submission.
Analysis Workflow
The general workflow of DNA/RNA Drug Melting Temperature (Tm) Testing Service is as follows:
1. Sample Preparation
Receive samples and adjust buffer conditions if necessary.
2. Denaturation Process
Gradually increase the temperature under controlled conditions to induce strand separation of double-stranded or secondary-structured nucleic acids.
3. Signal Monitoring
Record changes in absorbance, fluorescence, or thermal signals of the sample in real time as temperature increases.
4. Melting Curve Generation
Plot the relationship between temperature and signal variation.
5. Tm Value Calculation
Determine the melting temperature based on the curve inflection point or peak value.
6. Data Reporting
Provide a complete technical report including Tm values, experimental conditions, melting curves, and result interpretation.
Service Advantages
Advanced Analysis Platform: MtoZ Biolabs established an advanced DNA/RNA Drug Melting Temperature (Tm) Testing Service platform, guaranteeing reliable, fast, and highly accurate analysis service.
One-Time-Charge: Our pricing is transparent, no hidden fees or additional costs.
Reproducible Results: Strict temperature control systems and standardized procedures ensure accurate and reliable results.
Diverse Sample Compatibility: Applicable to various forms, including natural and modified nucleic acid drugs.
Sample Submission Suggestions
Sample Types
Supported samples include DNA, RNA, oligonucleotides (modified or unmodified), primers, siRNA, and aptamers.
Storage and Transportation
Recommended storage at low temperature (-20°C or below), with shipment on dry ice.
It is recommended to contact the MtoZ Biolabs technical team prior to sample submission to obtain detailed and tailored guidelines for sample preparation and submission.
Applications
Determine appropriate annealing temperatures to improve amplification efficiency and specificity.
Optimize hybridization conditions for experiments such as FISH and Southern blot.
Ensure siRNA stability and gene silencing efficiency.
Screen candidate drugs with better thermal stability through Tm determination.
Assist in guide RNA design to ensure reliable binding and cleavage.
Evaluate the impact of different buffer systems, ionic conditions, or modification types on the stability of nucleic acid drugs.
FAQ
Q1: How Is the Accuracy and Reproducibility of Tm Testing Results Ensured?
A1: All experiments are conducted under constant temperature conditions with instruments calibrated using reference standards. Each sample is tested in at least three parallel experiments, and mean values with standard deviations are provided to ensure reliable and traceable data. For critical projects, we recommend using dual-method verification to enhance credibility.
Q2: Do Buffer Conditions and Environmental Factors Affect Tm?
A2: Yes, Tm is significantly influenced by ion concentration, pH, and buffer systems. MtoZ Biolabs can set up simulated physiological environments or specific experimental conditions according to client requirements to ensure the data obtained is relevant for practical applications.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Tm Determination Results
4. Melting Curve Graphs
5. Data Analysis
6. Raw Data Files
Related Services
DNA/RNA Drug pKa Determination Service
DNA/RNA Drug Moisture Content Determination Service
DNA/RNA Drug Molar Extinction Coefficient Determination Service
How to order?







