DNA/RNA Drug Potency Assessment Service
- Candidate Drug Screening
- Pharmacodynamics and Mechanism Studies
- Quality Control and Batch Consistency
- Formulation Development and Stability Studies
- Regulatory Submission and Preclinical Research
DNA/RNA Drug Potency Assessment Service is designed to systematically evaluate the ability of nucleic acid drugs to exert their intended biological effects under specific conditions and to quantitatively characterize their dose-response relationships. Potency assessment provides scientific validation of the therapeutic potential of candidate drugs and supports dose optimization, quality control, formulation development, and regulatory submission, making it an indispensable core step in the research and application of nucleic acid drugs.
With the expanding use of novel nucleic acid drugs such as small interfering RNA (siRNA) and antisense oligonucleotides (ASOs) in fields including genetic diseases, cancer, and immune disorders, scientific potency assessment has become a critical component of their development and quality management. Nucleic acid drugs exert therapeutic effects through diverse mechanisms such as regulating gene expression, blocking translation, or promoting protein synthesis, and the strength of these effects directly determines dose selection, clinical efficacy, and safety. Without reliable potency assessment, there is a risk of underdosing or overdosing, as well as challenges in maintaining batch-to-batch consistency and meeting regulatory compliance. Therefore, systematic potency assessment of DNA/RNA drugs not only provides a scientific basis for drug screening and optimization but also supplies essential data for formulation stability studies, quality control, and international regulatory submission, serving as a key guarantee for the successful clinical and commercial development of nucleic acid drugs.
Services at MtoZ Biolabs
MtoZ Biolabs provides DNA/RNA Drug Potency Assessment Service to systematically evaluate the biological activity and therapeutic potential of nucleic acid drugs. By combining cell-based assays, target binding analyses, physicochemical characterization, and mass spectrometry platforms, we can comprehensively reveal the functional properties of drugs at multiple levels.
Cell-based assays are used to evaluate gene expression inhibition, protein level changes, and effects on cell proliferation or inhibition. Binding assays such as ELISA and SPR precisely measure drug-target affinity and binding kinetics. Physicochemical analyses, including molecular weight determination, electrophoresis, and chromatography, characterize structural features and modifications. Mass spectrometry platforms enable quantitative analysis of drug distribution and metabolite levels in complex biological matrices.
Through multi-platform integration and strict quality control, MtoZ Biolabs provides clients with highly sensitive and reliable potency assessment data to support nucleic acid drug development, quality control, and regulatory submission.
Analysis Workflow
The general workflow of DNA/RNA Drug Potency Assessment Service is as follows:
1. Sample Preparation
Receive and process DNA/RNA drug samples, with buffer exchange or stability treatment performed if necessary.
2. Experimental Design
Develop testing protocols based on the drug mechanism and research objectives.
3. Potency Testing
Conduct gene expression assays, protein level measurements, or binding assays to obtain activity data.
4. Data Collection and Curve Fitting
Record experimental results, generate dose-response curves, and calculate parameters such as EC₅₀ and IC₅₀.
5. Result Report
Provide a complete technical report including experimental conditions, raw data, and data analysis.
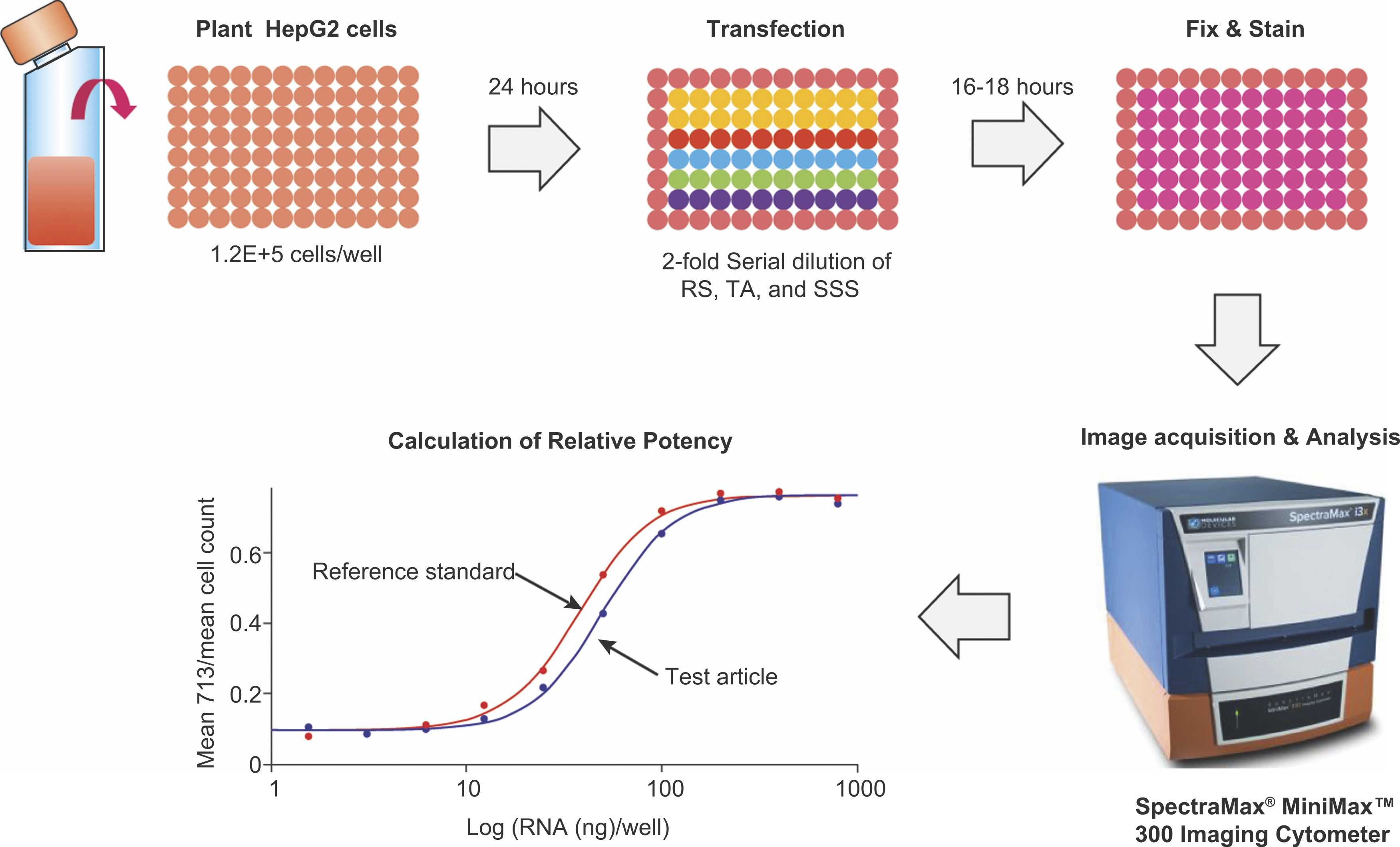
Li HH. et al. J Pharm Biomed Anal. 2023.
Figure 1. Schematic of the cell-based relative potency assay for RSV mRNA Vaccine V171.
Service Advantages
Advanced Analysis Platform: MtoZ Biolabs established an advanced DNA/RNA Drug Potency Assessment Service platform, guaranteeing reliable, fast, and highly accurate analysis service.
One-Time-Charge: Our pricing is transparent, no hidden fees or additional costs.
High-Data-Quality: Deep data coverage with strict data quality control. AI-powered bioinformatics platform integrates all DNA/RNA Drug Potency Assessment data, providing clients with a comprehensive data report.
Multidimensional Evaluation System: Integrates cell-based functional assays, target binding analyses, physicochemical characterization, and mass spectrometry to comprehensively reveal drug potency.
Customized Study Design: Provides personalized evaluation strategies based on drug type, mechanism of action, and development stage.
Sample Submission Suggestions
Sample Types
Applicable to siRNA, mRNA, ASOs, aptamers, and other modified nucleic acid drugs.
Storage and Transportation
Recommended storage at -20°C or lower, with shipment on dry ice to maintain sample stability.
It is recommended to contact the MtoZ Biolabs technical team prior to sample submission to obtain detailed and tailored guidelines for sample preparation and submission.
Applications
Rapidly evaluate whether molecules possess the expected biological activity.
Reveal drug action pathways and regulatory effects at the molecular level.
Ensure stability and reproducibility of drugs during research and production.
Compare the impact of different formulations on drug activity and optimize formulation processes.
Provide potency data compliant with international standards to support drug registration and regulatory review.
FAQ
Q1: What Methods Are Primarily Used in DNA/RNA Drug Potency Assessment Service?
A1: MtoZ Biolabs applies a combination of cell-based assays (such as cell signaling detection, cell migration assays, and cell proliferation/inhibition assays), target binding assays (ELISA, SPR), as well as physicochemical characterization and mass spectrometry analysis to comprehensively characterize the biological activity and mechanism of action of drugs.
Q2: Which Indicators Are Commonly Used to Present Potency Assessment Results?
A2: Results are typically presented as dose-response curves and quantified using key parameters such as EC₅₀ (half-maximal effective concentration), IC₅₀ (half-maximal inhibitory concentration), and Emax (maximum effect value) to reflect drug potency.
Related Services
DNA/RNA Drug Size and Aggregation Analysis Service
DNA/RNA Drug Quantification and Potency Determination Service
How to order?







