DNA/RNA Drug Impurity Profiling Service
MtoZ Biolabs provides a specialized DNA/RNA Drug Impurity Profiling Service to support the pharmaceutical industry in developing safe and effective nucleic acid therapeutics. Through advanced analytical platforms and expert bioanalysis, MtoZ Biolabs provides a comprehensive view of impurity types, their relative abundance, and their potential impact. This enables our clients to make informed decisions during drug development, manufacturing, quality control, and regulatory submission.
DNA and RNA therapeutics, including antisense oligonucleotides, small interfering RNAs (siRNA), messenger RNA (mRNA), aptamers, and plasmid-based therapies, are transforming modern medicine. They are applied in cancer treatment, genetic disease correction, vaccine development, and infectious disease control. However, due to their complex structures and chemical modifications, nucleic acid drugs are vulnerable to a wide range of impurities. These impurities may arise during synthesis, purification, formulation, storage, or even transport.
Impurity profiling involves the systematic detection, identification, and quantification of unwanted chemical or biological species present in a drug product. In DNA and RNA drugs, impurities can include truncated oligonucleotides, depurinated fragments, incomplete coupling by-products, modified nucleotides, double-stranded contaminants, residual solvents, heavy metals, endotoxins, and degradation products. Even trace levels of these impurities may impact safety, therapeutic efficacy, or stability, and therefore must be carefully monitored and controlled.
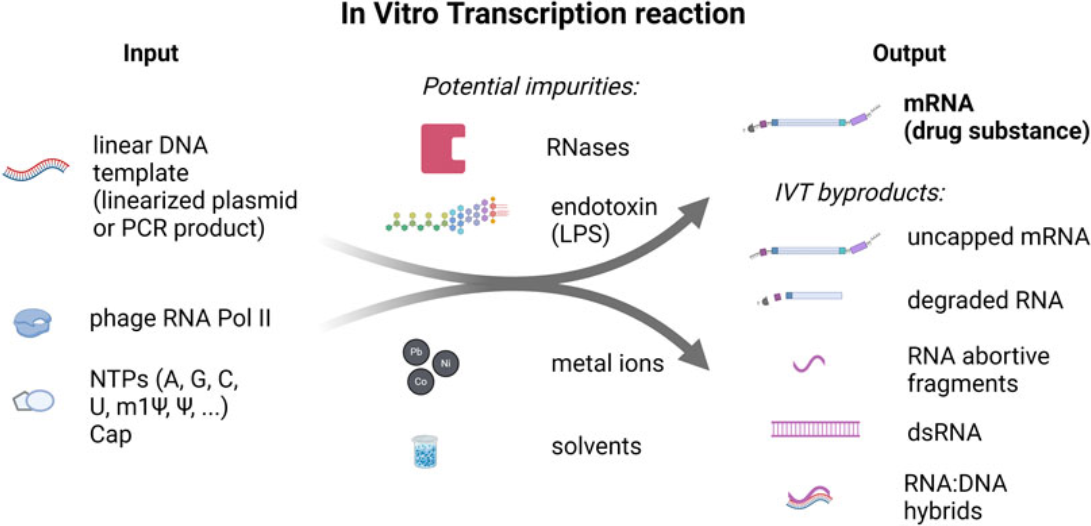
Figure1. In Vitro Transcription Reaction Input and Output Components and Potential Impurities
Service at MtoZ Biolabs
MtoZ Biolabs' DNA/RNA Drug Impurity Profiling Services help pharmaceutical developers ensure the safety, efficacy, and consistency of nucleic acid therapeutics. Using advanced analytical platforms such as liquid chromatography (LC), mass spectrometry (MS), and capillary electrophoresis (CE), we quantify and identify a wide range of impurities that can affect drug performance and regulatory compliance.
Our service offerings include:
💠Development of Drug-Specific Analytical Methods
We design and validate analytical strategies tailored to each DNA or RNA drug, addressing its unique chemical structure and formulation to ensure accurate impurity assessment.
💠Impurity Identification and Characterization
Leveraging high-resolution LC-MS and CE-based methods, we detect and structurally characterize impurities, including truncated oligonucleotides, modified nucleotides, and degradation products.
💠Impurity Quantification
We provide both relative and absolute quantification of impurities, enabling batch-to-batch consistency evaluation and supporting stability studies and regulatory documentation.
Analysis Workflow
1. Sample Preparation: Solid-phase extraction, enzymatic digestion, or precipitation methods are applied to prepare samples and isolate potential impuritie.
2. Custom Method Development: Analytical strategies are tailored based on drug characteristics, impurity types, and specific project requirements to ensure reliable detection and measurement.
3. Instrumental Analysis and Detection: Advanced platforms including liquid chromatography (LC), mass spectrometry (MS), and capillary electrophoresis (CE) are used to separate, detect, and profile impurities.
4. Impurity Identification and Characterization: Structural elucidation of impurities is performed to determine their origin and potential impact on drug safety and efficacy.
5. Impurity Quantification: Both relative and absolute quantification of impurities are conducted to assess batch consistency and compliance with quality standards.
6. Data Interpretation and Reporting: Results are compiled into a comprehensive report that integrates findings, provides expert interpretation, and outlines implications for product quality and regulatory submission.
Why Choose MtoZ Biolabs?
☑️Advanced analytical platforms ensuring high sensitivity and specificity
☑️Expert scientists with extensive experience in nucleic acid chemistry and drug analytics
☑️Customizable solutions aligned with project-specific and regional requirements
☑️Efficient turnaround times with reliable and reproducible results
☑️End-to-end support from project consultation to data interpretation
Applications
1. Drug Development and Method Optimization
Impurity profiling provides critical insights during the early stages of drug discovery and formulation. By identifying synthesis by-products and degradation pathways, researchers can refine drug design and optimize analytical methods to ensure high purity.
2. Manufacturing Process Quality Control
Routine impurity analysis helps monitor batch-to-batch consistency and detect process-related contaminants. This ensures that DNA and RNA drugs maintain safety and efficacy standards throughout production.
3. Regulatory Submission
Comprehensive impurity data supports documentation required by global health authorities. Profiling ensures that potential risks are assessed and controlled, strengthening the quality and safety evidence presented for approval.
4. Post-Marketing Quality Surveillance
Ongoing monitoring of impurities after drug launch helps detect changes caused by storage, distribution, or formulation modifications, ensuring continued compliance and safeguarding patient safety.
Sample Submission Suggestions
1. Sample Types
Acceptable samples include DNA drugs, RNA drugs, oligonucleotides (modified or unmodified), and aptamers.
2. Storage and Transportation
Samples should be stored at low temperature (-20°C or below), and shipment on dry ice is recommended.
It is recommended to contact the MtoZ Biolabs technical team prior to sample submission to obtain detailed and tailored guidelines for sample preparation and submission.
FAQ
Q1: Can you differentiate sequence-related impurities from chemical degradation products?
A1: Yes. By combining tandem mass spectrometry fragmentation data with chromatographic separation, we can distinguish sequence truncations, insertions, or substitutions from chemical modifications.
Q2: How do you ensure reproducibility in impurity profiling across different batches?
A2: Standardized workflows, system suitability tests, and use of internal standards help ensure consistent impurity profiling results, enabling reliable batch-to-batch comparison.
Deliverables
1. Detailed experimental methods and workflow
2. Instrumental parameters and materials used
3. Impurity profiles with chromatograms, electropherograms, and spectra
4. Identification and quantification of impurities
5. Data interpretation and conclusions
6. Raw data files
MtoZ Biolabs is committed to providing reliable, comprehensive, and high-quality analytical solutions to support every stage of DNA/RNA drug development. Free project evaluation, welcome to learn more details!
Related Services
DNA/RNA Drug Molecular Weight Confirmation Service
How to order?







