Carrier Capsid Protein Composition Analysis Service | DNA/RNA Drug
- Isolated Capsid Proteins: Recombinant or purified capsid protein fractions for sequencing, identification, and structural analysis.
- Formulated Drug Products: DNA or RNA drug formulations containing carrier capsid proteins as delivery components.
Carrier capsid proteins are the essential building blocks of viral vectors and nanoparticle-based delivery systems widely used in DNA and RNA drug development. MtoZ Biolabs provides Carrier Capsid Protein Composition Analysis Service to support pharmaceutical and biotechnology researchers in developing safe and effective DNA and RNA therapeutics.
Carrier capsid proteins possess an exceptional capacity for self-assembly, forming highly ordered structures that closely resemble viruses, known as virus-like particles (VLPs). VLPs can protect nucleic acids, promote their delivery into target cells, and influence biodistribution, immunogenicity, and therapeutic outcomes. The precise composition of carrier capsid proteins determines the safety, efficacy, and manufacturability of DNA and RNA drugs, making their characterization a critical component of drug development pipelines.
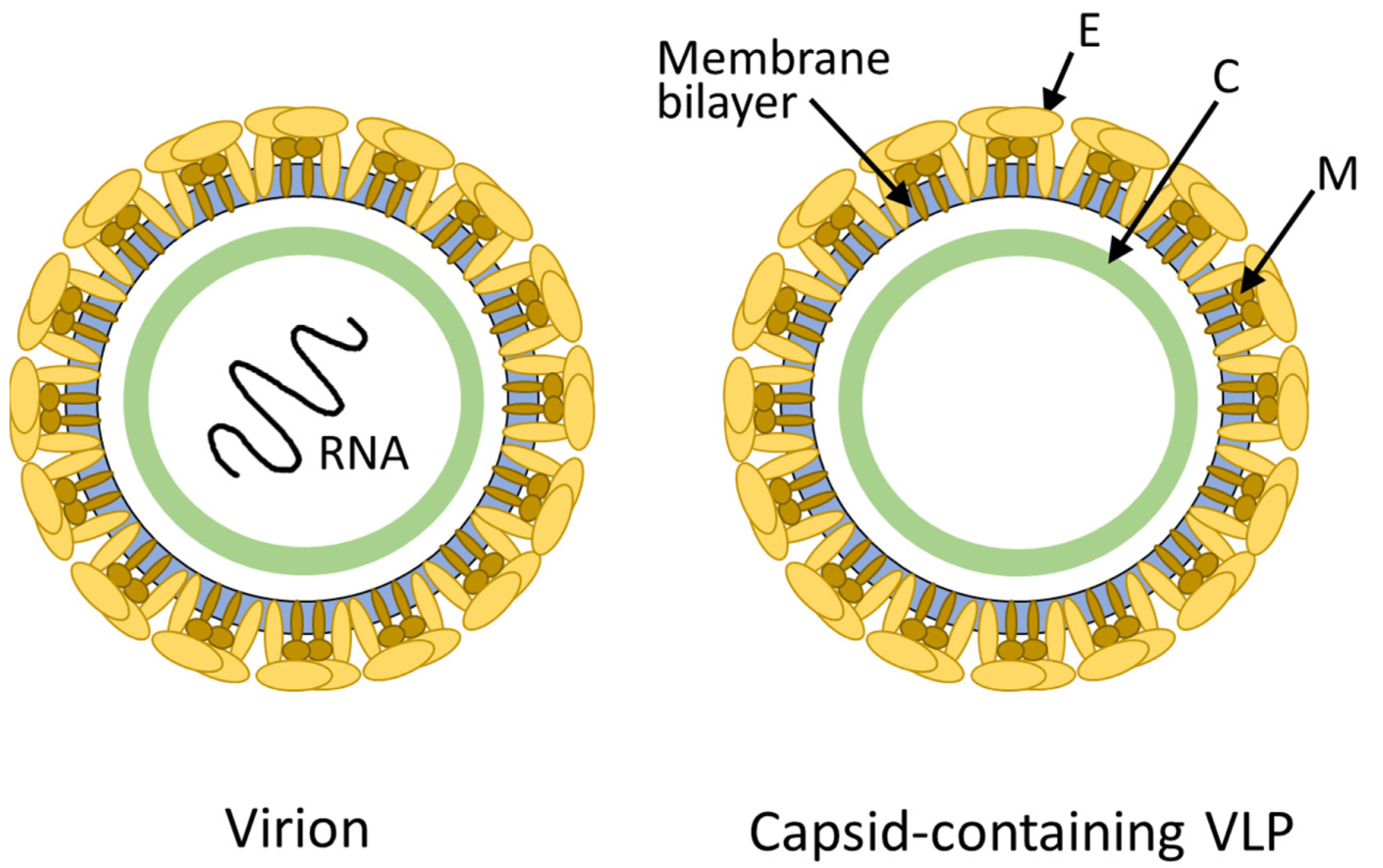
Figure 1. Schematic Diagrams of Infectious Virus (Virion) and Capsid-Containing Virus-Like Particle (VLP)
Protein composition analysis of carrier capsids involves determining the identity, abundance, and structural organization of capsid proteins, as well as detecting potential modifications, degradation, or impurities. Variability in capsid protein composition can directly impact the integrity of the delivery vehicle, affect drug encapsulation efficiency, and alter immune recognition. Comprehensive and accurate analysis ensures consistency across manufacturing batches, compliance with quality standards, and robust regulatory submissions.
Service at MtoZ Biolabs
With advanced analytical platforms and deep expertise in proteomics, MtoZ Biolabs provides accurate and reproducible characterization of capsid protein composition. Our insights help clients accelerate DNA and RNA drug development.
Our Carrier Capsid Protein Composition Analysis Services include:
We perform detailed sequencing of capsid proteins to confirm amino acid composition and detect possible sequence variants or modifications, ensuring accurate molecular identity.
💠Protein Identification and Quantification
Using state-of-the-art LC-MS/MS and complementary techniques, we identify all protein subunits within capsids and provide both relative and absolute quantification, enabling batch consistency and quality monitoring.
Through approaches such as circular dichroism spectroscopy, NMR, and computational modeling, we assess secondary and tertiary structural features of capsid proteins, providing insights into folding, stability, and assembly.
Our advanced bioinformatics pipelines integrate proteomics data to reveal structural organization, post-translational modifications, and correlations with functional performance, supporting comprehensive interpretation and regulatory submissions.
Analysis Workflow
Why Choose MtoZ Biolabs?
☑️We apply advanced platforms such as LC-MS/MS, NMR, and circular dichroism spectroscopy to deliver precise analysis of capsid protein composition.
☑️Our scientists have extensive expertise in proteomics, ensuring accurate sequencing, quantification, and structural insights.
☑️Workflows are customized to meet specific project needs and comply with regional regulatory requirements.
☑️Data quality is guaranteed through validated methods that provide reproducible and high-resolution results.
☑️We offer full-cycle support from study design to data interpretation, helping clients accelerate DNA and RNA drug development with confidence.
Applications
1. Drug Development and Formulation
Supports the design and optimization of DNA/RNA therapeutics by ensuring the integrity and stability of carrier capsids.
2. Manufacturing Quality Control
Provides routine monitoring of capsid protein composition to ensure batch-to-batch consistency.
3. Regulatory Submissions
Supplies validated analytical data packages required for approval of DNA/RNA therapeutics.
4. Comparability Studies
Assists in evaluating process changes, technology transfers, or biosimilar development by comparing protein composition across lots.
5. Stability Testing
Evaluates the impact of storage conditions, stress testing, and formulation changes on capsid protein integrity.
6. Biological Research
Provides mechanistic insights into capsid protein structure and function, supporting fundamental and applied research.
Sample Submission Suggestions
1. Sample Types
We accept a wide range of DNA and RNA drug-related samples, including but not limited to:
2. Storage and Transportation
Samples should be stored at -80℃ and shipped with dry ice
*Note: If you have special sample types or require additional guidance, please contact us for personalized support before sample preparation.
Deliverables
1. Comprehensive experimental details
2. Information on materials, instruments, and methods
3. Protein identity and composition profile
4. Quantitative analysis of capsid protein ratios
5. Structural organization insights
6. Raw data files and interpreted results
7. Final technical report with conclusions and recommendations
MtoZ Biolabs is committed to providing reliable, comprehensive, and high-quality Carrier Capsid Protein Composition Analysis Services to support DNA/RNA drug development. Free project evaluation, welcome to learn more details!
Related Services
DNA/RNA Drug Purity and Impurity Analysis Service
DNA/RNA Drug Molecular Weight Confirmation Service
How to order?







