VLP Encapsulation Efficiency Measurement Service | DNA/RNA Drug
- DNA and RNA drug delivery optimization
- Gene therapy vector development
- Vaccine formulation assessment
- Nanoparticle drug carrier evaluation
- Stability and release studies
- Quality control in manufacturing
Efficient drug delivery systems are vital for the success of DNA and RNA therapeutics. These systems ensure that fragile nucleic acids reach their target sites intact, achieve effective cellular uptake, and produce the intended therapeutic outcomes. Among the many delivery platforms explored, virus-like particles (VLPs) have emerged as one of the most promising carriers due to their natural architecture and ability to mimic viral functions without carrying infectious genetic material. MtoZ Biolabs provides VLP Encapsulation Efficiency Measurement Service to help pharmaceutical and biotechnology researchers optimize their DNA and RNA drug formulations.
VLPs are non-infectious nanoparticles built from viral proteins. These particles can encapsulate DNA or RNA, shield them from enzymatic degradation, and enhance delivery into target cells. Their inherent stability and tunable design make VLPs attractive candidates for drug delivery, vaccine development, and gene therapy applications. Encapsulation efficiency measurement determines the proportion of nucleic acids successfully packaged within VLPs compared to the total amount used during formulation. This measurement is critical because it directly influences therapeutic performance, manufacturing efficiency, and regulatory compliance. High encapsulation efficiency means that the delivery system is optimized to maximize nucleic acid protection and minimize loss, while low efficiency may compromise therapeutic efficacy and increase production costs.
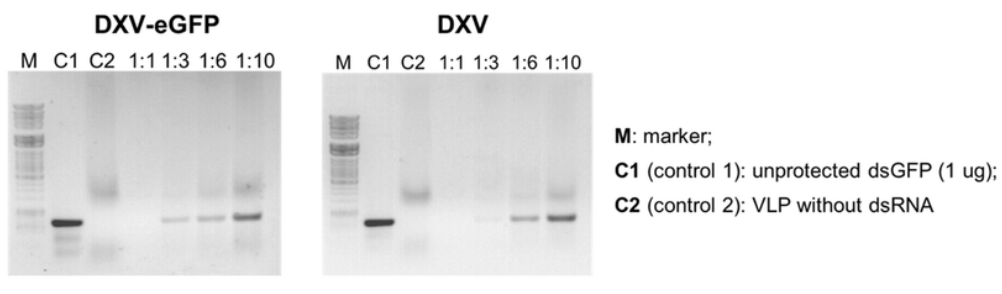
Figure 1. Analysis of the Encapsulation Efficiency at Different Molar Ratios of dsGFP to Drosophila X Virus-Like Particles (DXV-VLPs) by Agarose Gel Electrophoresis
Service at MtoZ Biolabs
MtoZ Biolabs offers comprehensive analytical solutions for evaluating the performance and quality of virus-like particle (VLP) systems in DNA and RNA drug development. With our advanced platforms and experienced scientific team, we deliver precise, reproducible, and regulatory-compliant data.
Our VLP Encapsulation Efficiency Measurement Service covers the following aspects:
💠VLP Characterization
We perform structural and compositional analysis of VLPs to confirm morphology, size distribution, protein composition, and homogeneity. This ensures the delivery system is well defined and suitable for nucleic acid encapsulation.
💠Encapsulation Efficiency Measurement
Using advanced techniques such as chromatography, capillary electrophoresis (CE), fluorescence-based quantification, and mass spectrometry, we determine the proportion of DNA or RNA molecules successfully packaged inside VLPs, enabling accurate evaluation of drug loading efficiency and batch-to-batch consistency.
💠Stability Testing
We evaluate VLP stability under different stress conditions including temperature, pH, and storage duration, providing critical data on shelf-life and formulation robustness.
💠Release Kinetics
We analyze the controlled release of DNA or RNA drugs from VLPs under physiological conditions. This provides valuable insight into delivery efficiency, therapeutic performance, and dosage optimization.
Analysis Workflow
1. Drug Encapsulation
The DNA or RNA drug is encapsulated within virus-like particles (VLPs) under optimized conditions to ensure efficient loading and structural stability.
2. Purification of Encapsulated VLPs
Encapsulated VLPs are separated from free nucleic acids and empty particles using purification techniques such as chromatography or ultrafiltration to obtain clean samples.
3. Quantification of Encapsulated Drug
Multiple analytical methods including chromatography, capillary electrophoresis (CE), fluorescence-based assays, and mass spectrometry are applied to measure the amount of nucleic acid successfully packaged inside the VLPs.
4. Encapsulation Efficiency Calculation
The ratio of encapsulated nucleic acids to the total drug input is calculated, providing a clear evaluation of encapsulation efficiency and consistency across batches.
Why Choose MtoZ Biolabs?
✅MtoZ Biolabs established an advanced VLP Encapsulation Efficiency Measurement Service platform, guaranteeing reliable, fast, and highly accurate analysis service.
✅Our team of experienced scientists specializes in DNA and RNA drug delivery systems, ensuring that every project benefits from deep technical knowledge and practical expertise.
✅We offer customized workflows tailored to the unique requirements of each drug candidate, enabling reliable evaluation of encapsulation, stability, and release kinetics.
✅Our services emphasize high efficiency and rapid turnaround times without compromising scientific rigor, supporting clients through every stage of drug development.
✅We provide end-to-end project support, from consultation and method design to result interpretation, ensuring smooth integration of encapsulation analysis into broader R&D pipelines.
Applications
Deliverables
1. Comprehensive experimental details and workflow documentation
2. List of materials, instruments, and analytical methods used
3. Raw and processed data from encapsulation efficiency measurements
4. Quantitative results of encapsulated vs. unencapsulated nucleic acids
5. Stability and release profile data (if included in the study)
6. Data interpretation and summary report with key conclusions
MtoZ Biolabs is committed to providing reliable, comprehensive, and high-quality VLP Encapsulation Efficiency Measurement Services to support DNA/RNA drug development. Free project evaluation, welcome to learn more details!
Related Services
DNA/RNA Drug Purity and Impurity Analysis Service
DNA/RNA Drug Molecular Weight Confirmation Service
How to order?







