Recombinant Collagen Biological Function Evaluation Service
Recombinant collagen has emerged as a next-generation biomaterial widely applied in tissue engineering, skin regeneration, aesthetic medicine, and drug delivery systems. Its high purity, tunable structure, and absence of animal-derived components make it an ideal candidate for clinical translation. However, a thorough biological function assessment is essential before advancing into preclinical or commercial development.
To meet this demand, MtoZ Biolabs offers standardized Recombinant Collagen Biological Function Evaluation Service tailored to both academic and industrial research. Utilizing a suite of in vitro assays, we quantitatively assess critical biological properties—including cell proliferation, cytotoxicity, migration, and adhesion—to evaluate the biocompatibility and functional potential of recombinant collagen samples.
Services at MtoZ Biolabs
As part of its Recombinant Collagen Biological Function Evaluation Service, MtoZ Biolabs has integrated four categories of standardized in vitro cellular assays to assess key aspects such as cell activity and material biocompatibility.
Cell Proliferation Assay Service | Recombinant Collagen Evaluation
Measures cell viability enhancement using the CCK-8 method to assess the collagen’s pro-proliferative capacity.
Cytotoxicity Assay Service | Recombinant Collagen Evaluation
Uses the MTT assay to determine cellular toxicity levels from collagen extracts, reflecting material safety.
Cell Migration Assay Service | Recombinant Collagen Evaluation
Evaluates migration behavior through scratch or Transwell assays, indicating tissue repair potential.
Cell Adhesion Assay Service | Recombinant Collagen Evaluation
Quantifies cell attachment efficiency on collagen surfaces using fluorescence-based assays to assess scaffold performance.
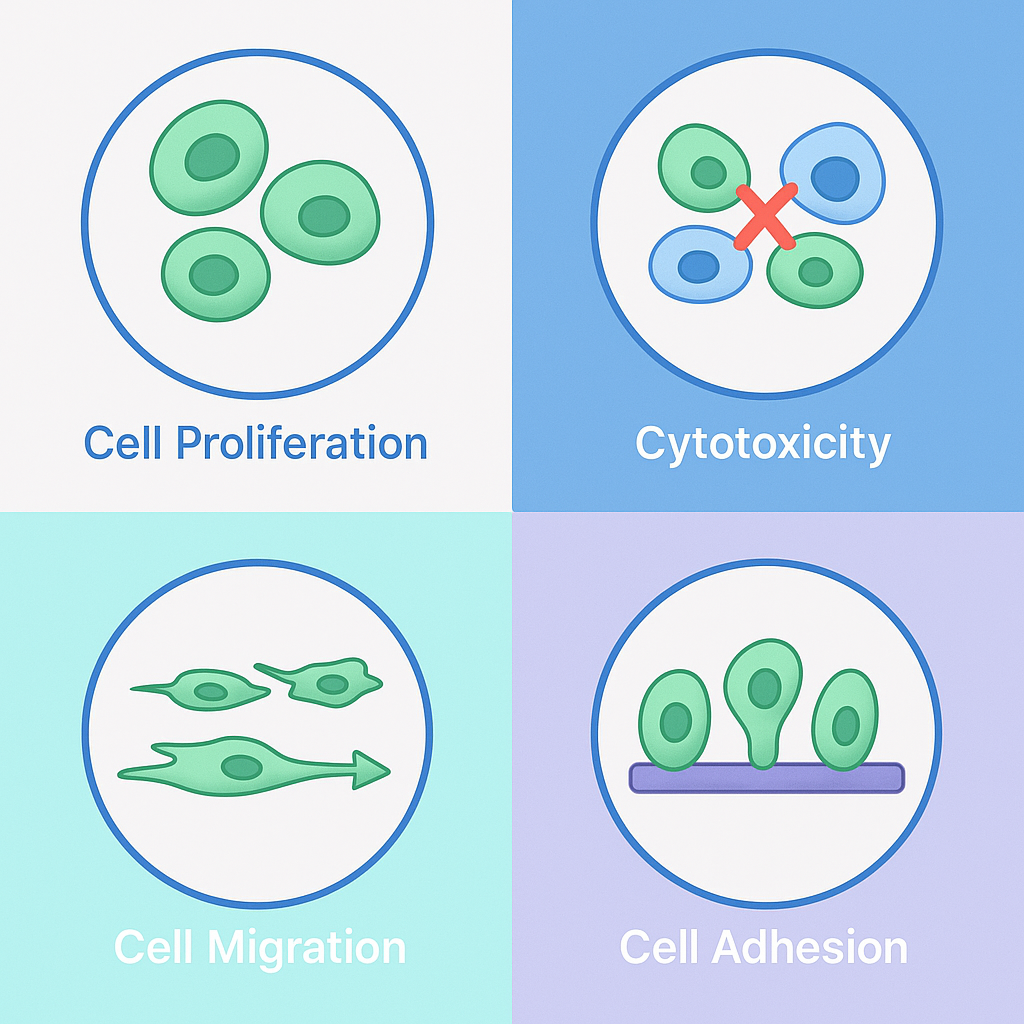
Figure 1. Core Cellular Assays for Recombinant Collagen Functional Evaluation
All services are available in standard and customizable formats, compatible with various collagen types (e.g., type I, type III), and support multi-batch testing for inter-sample comparison.
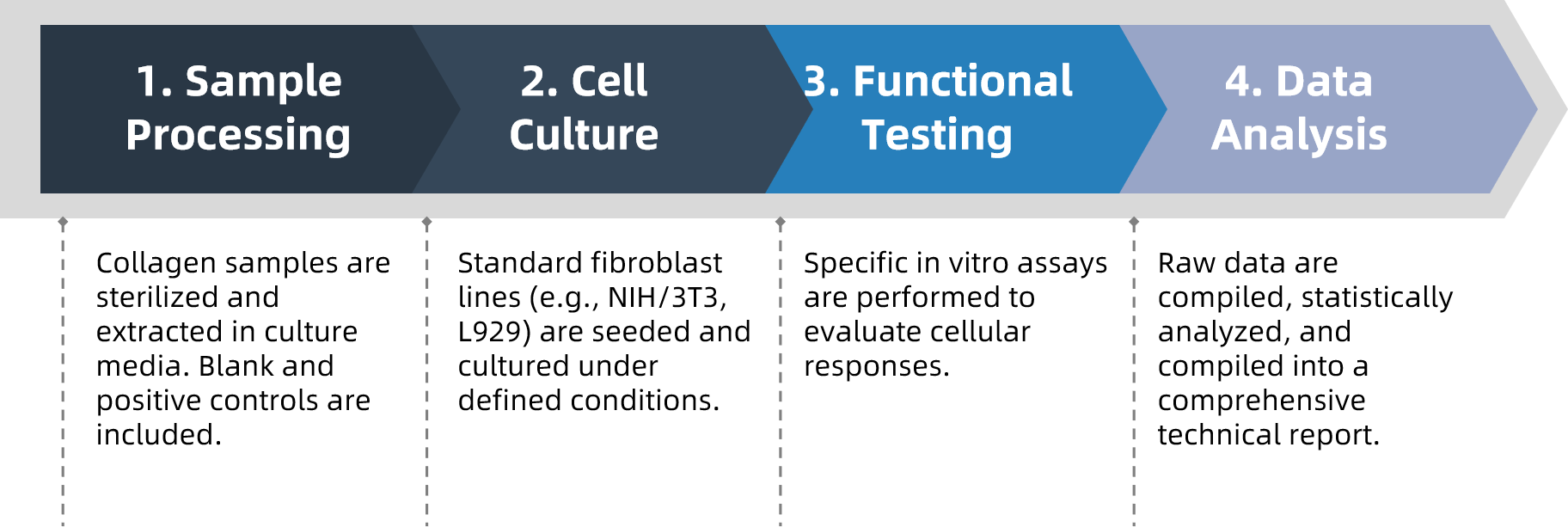
Analysis Workflow
To ensure consistency and traceability, MtoZ Biolabs follows a well-defined experimental pipeline:
Why Choose MtoZ Biolabs?
✔ Multi-dimensional Evaluation: Comprehensive assessment of biological functions: proliferation, cytotoxicity, migration, and adhesion.
✔ Standardized & Reproducible Protocols: SOP-driven workflows ensure data reliability and cross-batch comparability.
✔ Flexible Experimental Design: Customizable cell lines, concentration gradients, and incubation timelines to match your study goals.
✔ High Sample Compatibility: Supports various forms and modifications of recombinant collagen (lyophilized or in solution).
✔ Detailed & Traceable Reporting: Integrated delivery of imaging, numeric data, statistics, and annotated conclusions for publication or regulatory submission.
✔ One-Time-Charge: Our pricing is transparent, no hidden fees or additional costs.
Sample Submission Suggestions
To guarantee optimal results, please adhere to the following submission guidelines:
· Sample Form: Acceptable formats include lyophilized powder or clarified solution (free from visible precipitate).
· Suggested Volume: Submit ≥1 mL per assay or equivalent powder amount, depending on test parameters.
· Storage & Transport: Samples should be sealed and shipped under low-temperature, light-protected conditions (4°C or –20°C). Avoid freeze–thaw cycles.
· Documentation: Please indicate sample ID, concentration, solvent, and intended assays. Providing product background/formulation details is encouraged to support protocol optimization.
If you need assistance with sample preparation or submission, our technical support team is ready to help ensure a smooth evaluation process.
Applications
MtoZ Biolabs' Recombinant Collagen Biological Function Evaluation Service is widely applicable across various sectors within life sciences and biomaterials, offering dependable technical support for research, development, and quality control:
· Tissue Engineering & Regenerative Medicine: Assess the biological functionality of collagen-based scaffolds or biomaterials in promoting cell adhesion, migration, and tissue repair.
· Biomedical Material Development: Evaluate the cytocompatibility and safety of recombinant collagen used in wound dressings, hemostatic agents, and dermal substitutes.
· Cosmetics & Functional Skincare: Analyze the potential of collagen-derived ingredients to enhance skin cell proliferation and improve extracellular matrix structure.
· Biopharmaceuticals: Validate the biosafety and functional performance of recombinant collagen used in culture media formulations, drug delivery systems, and pharmaceutical excipients.
· Academic & Basic Research: Support researchers in investigating the cellular effects of collagen, offering robust data for mechanistic and translational studies.
What Could be Included in the Report?
1. High-resolution microscopic images (cell morphology, density, adhesion status)
2. Raw and summarized functional data (OD values, viability rates, migration distances, adhesion counts)
3. Statistical graphs and executive summary of findings
4. Complete documentation of assay conditions and technical protocols
Related Services
Recombinant Collagen Analysis Service
Recombinant Collagen Identification Service
Recombinant Collagen Structure Characterization Service
Recombinant Collagen Physicochemical Characterization Service
How to order?







