Recombinant collagen physicochemical characterization service
- Biomedical Implants & Scaffolds: Ensuring material compliance for use in tissue engineering or orthopedic matrices.
- Wound Healing Formulations: Optimizing collagen bioactivity and stability for medical dressings or injectables.
- Cosmetic Product Development: Evaluating purity and stability for anti-aging creams, serums, and dermal fillers.
- Academic and Translational Research: Supporting material reproducibility in collagen-based biological experiments.
- We accepts a variety of sample types, including purified recombinant collagen, lyophilized powders, and more.
- Samples should be shipped at low temperatures and protected from repeated freeze-thaw cycles.
- Lyophilized powders should be stored in a dry, sealed container and protected from moisture.
Recombinant collagen, produced via engineered expression systems, is widely used in regenerative medicine, tissue engineering, and cosmetic applications due to its consistency and reduced immunogenicity. Recombinant Collagen Physicochemical Characterization Service provides comprehensive analysis of its physical and chemical properties to ensure product quality, stability, and safety.
Collagen is the most abundant structural protein in the extracellular matrix (ECM) of human and mammalian tissues. It plays a pivotal role in maintaining tissue architecture, mechanical integrity, and cell–matrix interactions. To date, at least 28 distinct collagen types have been identified, among which types I, II, and III are most prominent in connective tissues such as skin, bone, cartilage, and tendons. The hallmark of collagen is its unique triple-helical structure, formed by three polypeptide α-chains with repeating Gly–X–Y sequences (where X and Y are frequently proline and hydroxyproline), contributing to its exceptional tensile strength and stability. Due to its excellent biocompatibility, low immunogenicity, and bioactivity, collagen has emerged as a critical biomaterial for applications in wound healing scaffolds, soft tissue fillers, drug delivery systems, and cosmetic formulations. However, sourcing native collagen from animal tissues often introduces challenges related to batch variability, immunogenic residues, potential pathogen transmission, and ethical concerns. These limitations have prompted a paradigm shift toward recombinant collagen which is biosynthetically produced using genetically engineered microbial or mammalian expression systems.
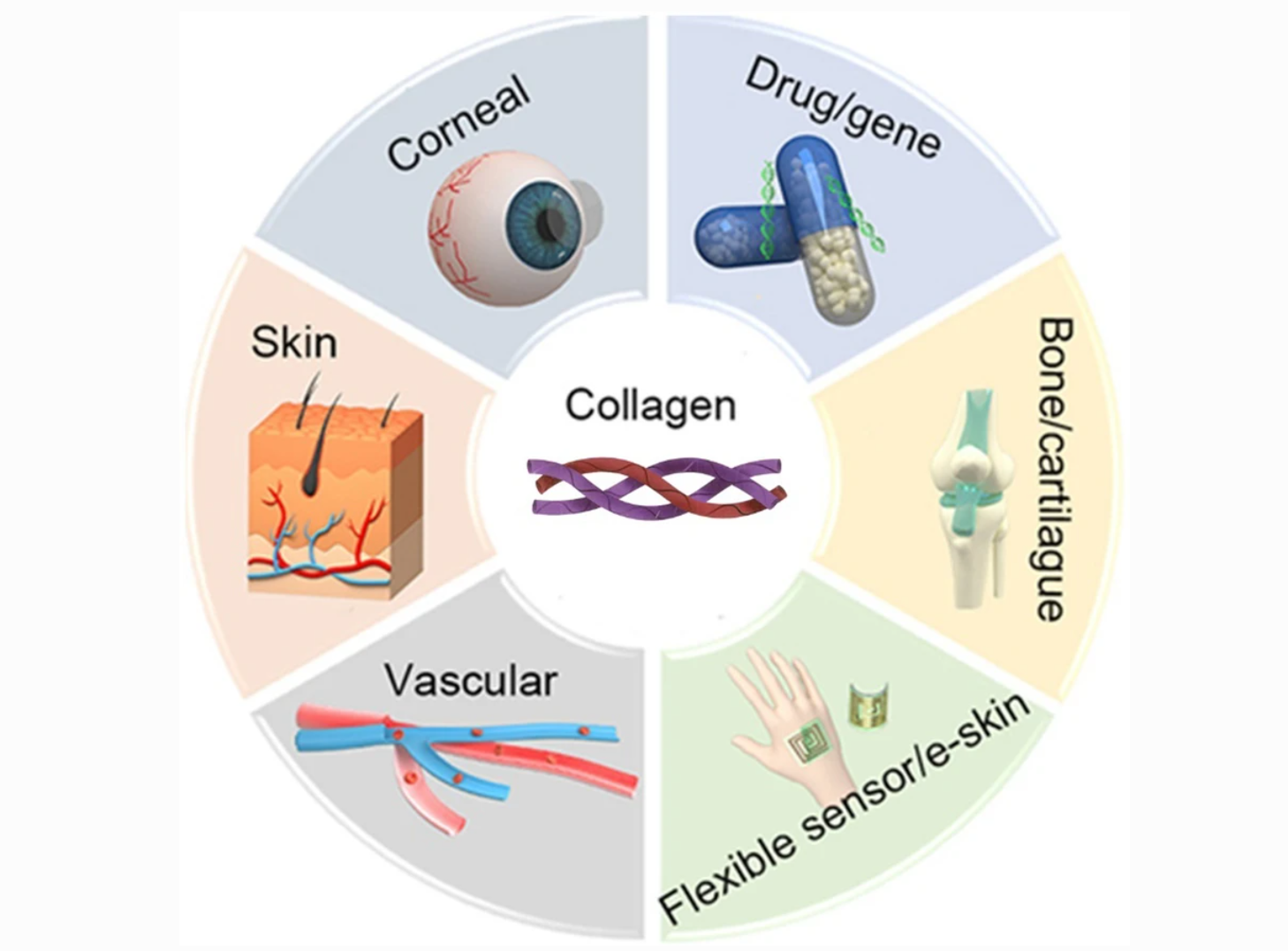
Figure 1. Various Collagen-Based Biomedical Applications
Recombinant collagen offers distinct advantages in terms of structural tunability, compositional consistency, and functional customization. Nonetheless, ensuring its equivalence to native collagen in terms of structural fidelity and physicochemical performance requires comprehensive characterization. Critical quality attributes such as moisture content, heavy metal and trace element levels, isoelectric point (pI), pH, thermal stability, purity, and total recombinant collagen content must be systematically assessed. These parameters directly influence the solubility, stability, functionality, and safety of the final product—particularly in clinical, pharmaceutical, and cosmetic applications.
Service at MtoZ Biolabs
MtoZ Biolabs offers a dedicated Recombinant Collagen Physicochemical Characterization Service that integrates advanced analytical platforms with standardized methodologies to deliver precise, reproducible data. Our workflows are designed to meet the rigorous demands of both academic and industrial clients, supporting R&D, product development, and regulatory compliance. MtoZ Biolabs specializes in providing high-resolution biochemical and biophysical analysis services for protein-based biomaterials. Leveraging our state-of-the-art instrumentation—including Thermo Scientific Orbitrap mass spectrometers, ICP-MS, UV-Vis spectrophotometers, HPLC, and DSC analyzers—we offer a comprehensive one-stop solution for characterizing recombinant collagen at multiple levels.
Quality Attributes We Characterize
Water content can significantly affect collagen’s stability, processing behavior, and shelf life. We quantify residual moisture levels, ensuring compliance with storage and formulation guidelines.
2. Heavy Metal and Trace Element Analysis
Trace metal contaminants may be introduced during the expression, purification, or formulation of recombinant collagen, potentially affecting product safety and efficacy. We use Inductively Coupled Plasma Mass Spectrometry (ICP-MS) to detect and quantify elemental residues with high sensitivity and accuracy, ensuring compliance with regulatory requirements.
3. Isoelectric Point (pI) Determination
The isoelectric point affects solubility, precipitation, and binding behavior. Capillary isoelectric focusing (cIEF) or isoelectric focusing gels are employed to determine the pI with high resolution, aiding downstream purification and formulation processes.
The native pH of collagen solutions is a key parameter affecting solubility, charge state, and bioactivity. We use precision pH meters with low-volume electrodes to determine accurate solution pH under controlled temperature and ionic conditions.
Thermal stability is assessed through Differential Scanning Calorimetry (DSC) to measure the collagen denaturation temperature (Td). This parameter reflects the triple-helix integrity and is critical for predicting shelf-life and application in thermally sensitive environments.
6. Recombinant Collagen Purity Analysis
Protein purity is evaluated using SDS-PAGE with densitometric analysis, as well as reverse-phase HPLC (RP-HPLC). These techniques help identify degradation products, truncations, or impurities arising from the expression host or purification process
7. Recombinant Collagen Content Analysis
We quantify recombinant collagen using mass spectrometry-based absolute quantification. Signature peptides are selected, synthesized as standards, and used to construct calibration curves for accurate measurement. This ensures reliable dosing for biomedical and cosmetic applications.
Service Advantages
☑️Advanced Analysis Platform: MtoZ Biolabs established an advanced Recombinant Collagen Physicochemical Characterization Service platform, guaranteeing reliable, fast, and highly accurate analysis service.
☑️One-Time-Charge: Our pricing is transparent, no hidden fees or additional costs.
☑️High-Data-Quality: All analyses undergo rigorous quality control and replicate validation, ensuring data accuracy, consistency, and scientific reliability across batches.
☑️Customizable Analysis Service: We offer flexible, modular service options tailored to your sample type, development stage, or regulatory needs—whether you need a single parameter test or a full characterization suite.
Applications
Our recombinant collagen characterization service supports diverse applications across life science and biomedical sectors:
Sample Submission Suggestions
If your samples require special handling, please contact us. We will provide customized submission instructions and pretreatment recommendations based on the sample type and analytical requirements.
Deliverables
1. Full documentation of test methods, Instruments and parameters used
2. Raw data and processed graphs
3. Quality Control Assessment
4. Data Analysis, Preprocessing, and Interpretation
How to order?







