Cytotoxicity Assay Service | Recombinant Collagen Evaluation
Evaluating the biocompatibility of recombinant collagen and related biomaterials is essential during early-stage development. Among in vitro tests, cytotoxicity analysis offers critical insight into whether a material suppresses cell viability, serving as a fundamental reference for downstream in vivo validation and clinical translation.
MtoZ Biolabs offers high-precision Cytotoxicity Assay Service utilizing the well-established MTT method to quantify the cytotoxic effects of recombinant collagen samples. By evaluating their impact on fibroblast viability following extract exposure, this service provides highly interpretable results through a standardized workflow—ideal for safety assessment, product screening, and regulatory submissions in medical, cosmetic, and tissue engineering applications.
Technical Principles
Our Cytotoxicity Assay Service is grounded in the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) method, a colorimetric technique widely used to evaluate cellular metabolic activity.
In this assay, metabolically active cells reduce the yellow MTT reagent to insoluble purple formazan crystals via mitochondrial dehydrogenase enzymes. The amount of formazan formed is directly proportional to the number of viable cells. After solubilizing the crystals, absorbance at 570 nm is measured using a microplate reader. A decrease in optical density (OD) indicates a reduction in cell viability, allowing precise quantification of cytotoxic effects. By comparing OD values across treatment groups—including blank and positive controls—we determine relative cell viability and grade cytotoxicity severity according to national biocompatibility standards.
Analysis Workflow
To ensure high-quality, reproducible results, MtoZ Biolabs follows a rigorously defined assay procedure covering all stages from sample handling to data interpretation:
1. Sample Preparation
Recombinant collagen samples are extracted using culture medium and filtered to remove particulate matter. Both blank controls (culture medium only) and positive controls (standard collagen samples) are included to benchmark results.
2. Cell Culture
L929 mouse fibroblast cells are cultured under standard conditions (37 °C, 5% CO₂) and seeded in 96-well plates at a density of 1×10⁵ cells/mL to ensure uniform attachment and growth.
3. Treatment and MTT Staining
Cells are exposed to collagen extracts for 24 hours, after which MTT solution is added. Following further incubation, formazan is dissolved using organic solvent, and OD values are recorded.
4. Data Interpretation
Relative cell viability is calculated for each treatment group. In parallel, microscopy is used to assess morphological changes. According to biocompatibility standards, samples with cytotoxicity scores greater than Grade 2 are considered toxic.

Why Choose MtoZ Biolabs?
✅ Standardized Cell Model System
Utilizes L929 fibroblast cells known for reproducible responses and sensitivity in toxicology testing.
✅ Robust and Reproducible SOPs
Every step is governed by validated SOPs to ensure consistency, minimize variability, and enable study replication.
✅ Comprehensive Readouts
Combines quantitative viability data (OD values) with categorical toxicity grading and visual observations.
✅ Flexible Sample Compatibility
Supports both lyophilized powders and liquid forms of recombinant collagen, with no solubility restrictions.
✅ Tailored Experimental Design
Customizable variables such as treatment duration, dilution gradients, and sample preparation protocols to meet diverse regulatory or research needs.
Applications
MtoZ Biolabs’ Cytotoxicity Assay Service is widely applicable in the preclinical assessment of recombinant collagen for diverse biomedical and commercial applications:
· Safety evaluation of collagen-based medical devices and scaffolds
· Preclinical screening of cosmetic formulations and skincare ingredients
· Quality control testing for tissue engineering matrices and hydrogels
· Supporting data for regulatory registration and R&D pipeline optimization
Sample Results
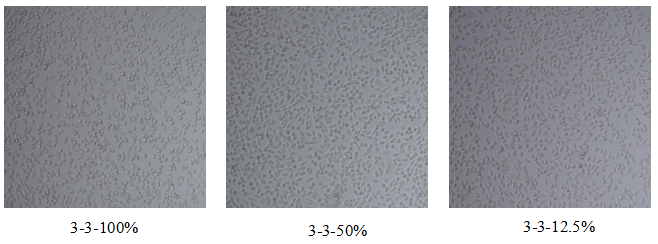
Figure 1. Microscopic Assessment of Cell Condition
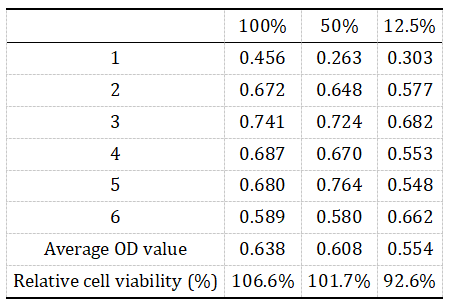
Figure 2. OD Values and Cell Survival Rates
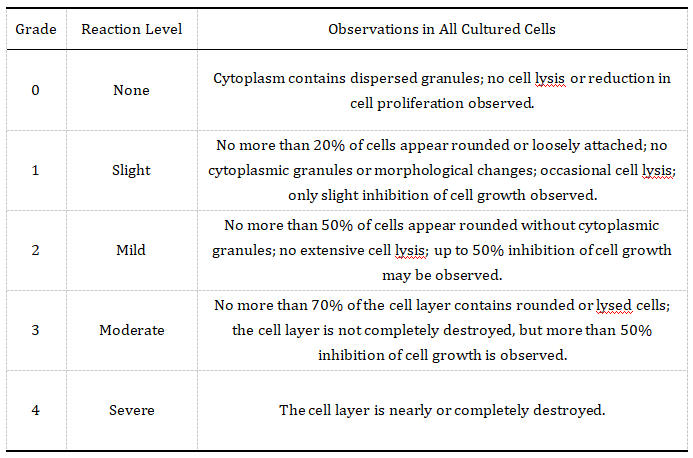
Figure 3. Qualitative Toxicity Rating (a grade >2 indicates cytotoxicity)
Complementary In Vitro Services
To enable multidimensional biological evaluation, MtoZ Biolabs also offers an integrated portfolio of cell-based functional assays, including:
These services can be bundled with Cytotoxicity Assay Service to construct a comprehensive safety and performance validation framework for recombinant collagen or other biomaterials.
Deliverables
1. Quantitative viability data across test and control groups (OD tables, bar charts)
2. Cytotoxicity scoring and qualitative assessment
3. Cell morphology images from representative wells
4. Experimental protocol and instrument parameters for reproducibility
5. Custom interpretation and consultation, if requested
To further customize your project or request a protocol, please feel free to contact the MtoZ Biolabs team of professionals. We are committed to providing comprehensive and credible data to support your material safety research and product development.
Related Services
Recombinant Collagen Biological Function Evaluation Service
Cell Proliferation Assay Service | Recombinant Collagen Evaluation
Cell Migration Assay Service | Recombinant Collagen Evaluation
Cell Adhesion Assay Service | Recombinant Collagen Evaluation
How to order?







