Recombinant Collagen Impurities Profile service
As recombinant collagen finds increasing applications in medical aesthetics, tissue engineering, and biopharmaceutical products, its quality and safety have become central concerns in research and product approval. Beyond sequence consistency and functional validation, the presence of impurities (such as host cell proteins, residual DNA, antibiotics, endotoxins, heavy metals, etc.) directly affects the product's stability, immunogenicity, and clinical safety.
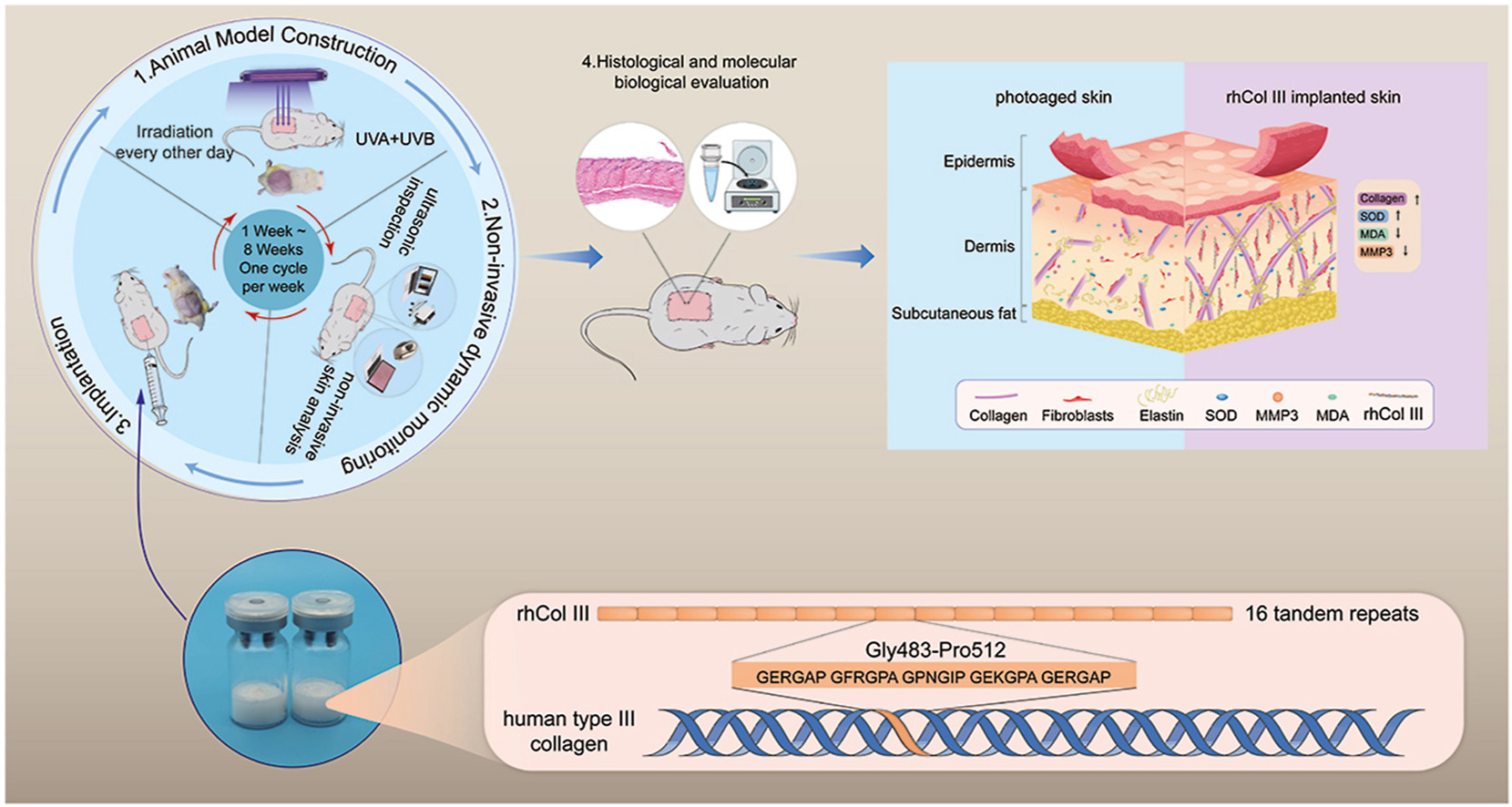
Wang, J. et al. Bioact. Mater. 2022.
Figure 1. Recombinant Collagen Animal Model and Application in Skin Tissue Engineering
MtoZ Biolabs offers Recombinant Collagen Impurities Profile Service which focuses on identifying and quantifying key impurities in recombinant collagen products. Combining high-resolution mass spectrometry, chromatography, molecular detection, and protein analysis platforms, this service delivers precise, reliable, and compliant impurity profiling data to support your product development.
Services at MtoZ Biolabs
MtoZ Biolabs' Recombinant Collagen Impurities Profile Service focuses on the five key impurities most commonly encountered in recombinant collagen development. We use reliable detection platforms to offer quantifiable and comparable results for various impurities.
1. Bacterial Endotoxin Testing
We use highly sensitive, standardized commercial kits (LAL method) to evaluate potential endotoxin contamination in your sample, applicable to solutions or finished products.
2. Host Cell DNA Testing
Using fluorescence quantitative kits (qPCR principle), we detect trace amounts of host-derived DNA residues, applicable across different expression systems.
3. Host Cell Protein (HCP) Testing
Combining ELISA (direct high-throughput screening) and LC-MS/MS (high-resolution identification and quantification), we comprehensively cover HCP residue risks across common expression systems (e.g., E. coli, CHO, Pichia).
4. Heavy Metals and Trace Elements Content Analysis
Using high-sensitivity inductively coupled plasma mass spectrometry (ICP-MS), we detect metal impurities (such as iron, zinc, copper, lead, nickel) and ensure compliance with medical-grade material control limits.
5. Kanamycin Residue Testing
We efficiently quantify potential kanamycin residues using enzyme-linked immunosorbent assay (ELISA), ensuring no antibiotic contamination risk, suitable for process residue verification or finished product testing.
Analysis Workflow
1. Sample Receipt and Information Registration: Confirm the sample’s background, batch number, and the testing parameters to be analyzed.
2. Pretreatment and Concentration Assessment: Perform necessary sample preparation steps, including buffer exchange and protein concentration determination, to ensure sample quality.
3. Method Selection and Platform Setup: Based on project requirements, select the appropriate detection methods and equipment, and implement quality control at multiple stages to ensure the reliability of the data.
4. Data Analysis and Impurity Quantification: Analyze multi-dimensional data to identify the types of impurities and determine their concentrations.
5. Standard Report Delivery: Provide a structured testing report, along with traceable raw data, to ensure transparency and reproducibility.

Service Advantages
✅ Comprehensive Multi-dimensional Detection Platform: Equipped with LC-MS/MS, ELISA, PCR, ICP-MS, and other mainstream platforms, we enable multi-method collaboration for precise and comprehensive impurity testing.
✅ Comprehensive Impurity Type Coverage: We focus on five key impurities and offer flexible modules for various research purposes, ensuring detailed analysis for different sample types.
✅ Expression System Compatibility: We have extensive experience with various expression systems (E. coli, yeast, mammalian cells) and provide tailored solutions for each.
✅ Clear and Stable Workflow: Our processes include batch and inter-batch quality control points, ensuring data reliability and consistency.
✅ Advanced Data Processing Support: We employ cutting-edge algorithms to enhance the accuracy of impurity identification, especially for low-abundance contaminants.
Sample Submission Suggestions
|
Sample Type |
Recommended Quantity |
Description |
|
Recombinant Collagen Solution |
≥ 500 μL (≥ 1 mg/mL) |
Please provide sample batch number and purification process info. |
|
Lyophilized Powder |
≥ 1 mg |
Accepts finished or intermediate product in powder form. |
Note: MtoZ Biolabs accepts samples from various expression systems and offers buffer exchange and pre-processing services. Please refer to our Sample Submission Guide for technical advice.
Applications
Our Recombinant Collagen Impurities Profile Service is suitable for a range of research and development applications:
· Screening and process development for recombinant collagen samples
· Impurity evaluation of final products and batch consistency verification
· Monitoring quality trends in material research
· Identifying impurity risks in new expression systems or purification processes
· Academic research on the relationship between protein impurities and performance
Deliverables
1. Impurity detection summary (with quantification data, detection limits)
2. Detection spectra (mass spectra, chromatograms, standard curves)
3. Metho descriptions and platform summaries
4. Sample information records and batch numbers
5. Full PDF testing reports and raw data file packages
MtoZ Biolabs is committed to providing comprehensive support for recombinant collagen impurity identification, helping you better understand product composition and quality trends. If you need more detailed service plans, sample submission advice, or project evaluations, please feel free to contact our technical team.
Related Services
How to order?







