Recombinant Collagen Host Cell DNA Testing Service
Recombinant collagen has gained wide adoption in biomedicine, drug development, and therapeutic applications. However, during production, residual host cell DNA can compromise the purity and safety of the final product. These DNA fragments may affect the biological activity of collagen or trigger immune responses, thereby posing clinical safety concerns. Therefore, detecting host cell DNA is a critical step in ensuring that recombinant collagen meets the standards for biomedical use.
MtoZ Biolabs provides Recombinant Collagen Host Cell DNA Testing Service using quantitative PCR (qPCR) kits to accurately detect trace amounts of host cell DNA in recombinant collagen samples. We ensure each batch is analyzed under strict safety standards, helping our clients guarantee product purity and biosafety across a range of application scenarios.
Analysis Workflow
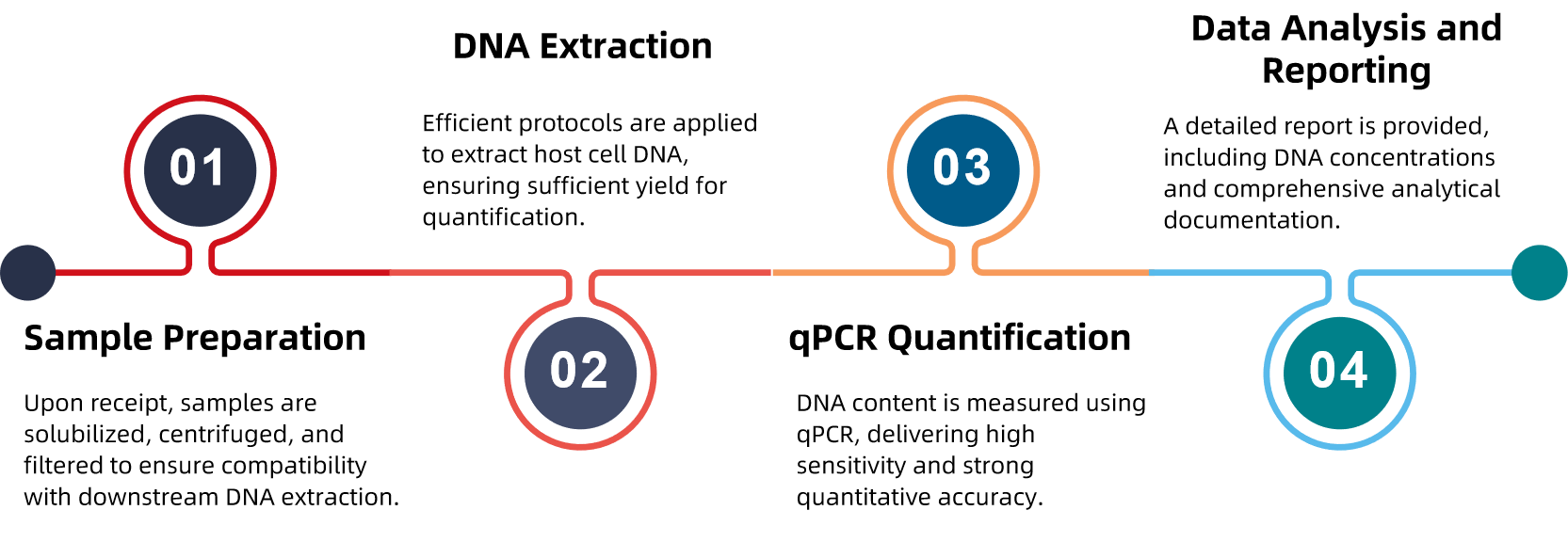
Why Choose MtoZ Biolabs?
✅ High Sensitivity and Accuracy: Leveraging qPCR kits, we detect extremely low levels of residual host cell DNA to meet stringent biosafety requirements.
✅ Broad Sample Compatibility: Applicable to various sample formats including purified recombinant collagen, lyophilized powders, and intermediate products.
✅ Rapid Turnaround: Results are typically delivered within 2–3 weeks, ensuring reliable data within your project timeline.
✅ One-Time-Charge: Our pricing is transparent, no hidden fees or additional costs.
Sample Submission Suggestions
|
Sample Type |
Recommended Quantity |
Notes |
|
Collagen solution |
≥500 μL (≥1 mg/mL) |
Avoid repeated freeze-thaw cycles; indicate purification stage and buffer conditions. |
|
Lyophilized powder |
≥5 mg |
Ensure dryness; avoid moisture exposure. |
Shipping & Storage Recommendations
Liquid Samples: Ship at 4 °C with cold packs; store at –20 °C upon arrival.
Lyophilized Samples: Ship on dry ice; store at room temperature in a dry, dark environment.
Note: We accept various sample types and offer optional pretreatment services. Contact our technical support team for specific guidance.
Applications
· Biopharmaceutical Quality Control: Detection of host cell DNA ensures that recombinant collagen products meet safety standards.
· Preclinical Research: Confirming DNA-free status is essential when using collagen in clinical research and therapeutic studies.
· Tissue Repair: Collagen used in wound healing must be verified to be free from host DNA to ensure safety.
· Drug Delivery Systems: When used as a drug delivery carrier, recombinant collagen must be DNA-free to avoid immune activation.
FAQ
Q1: How can host cell DNA be distinguished from other DNA contaminants in complex samples?
In complex matrices, other DNA sources may exist. At MtoZ Biolabs, we employ highly specific primers and probes along with multiplex PCR strategies to target only the host cell DNA. Optimized reaction conditions and highly sensitive detection protocols ensure specificity and accuracy.
Q2: How do you manage potential PCR inhibition in DNA quantification?
PCR inhibitors can lower detection sensitivity. We use optimized DNA extraction protocols to eliminate inhibitory substances (e.g., organic matter, salts). An internal standard method is applied to calibrate the inhibition effect. Our validated kits and PCR protocols are robust against interference, ensuring high accuracy across all stages.
To learn more about our Recombinant Collagen Host Cell DNA Testing Service, please reach out to the MtoZ Biolabs team. We are committed to providing expert-level technical support and quality assurance for your recombinant collagen development.
Related Services
How to order?







