Recombinant Collagen Host Cell Protein (HCP) Testing Service
- Recombinant Collagen Solution: ≥ 500 μL at ≥ 1 mg/mL; please include batch number and purification process information.
- Lyophilized Powder: ≥ 1 mg; both intermediate and final products are accepted.
Recombinant collagen, widely used in biopharmaceuticals, regenerative medicine, and medical device development, must meet strict safety and quality standards to ensure clinical efficacy and patient safety. One of the key quality attributes in the production of recombinant proteins is the residual level of host cell proteins (HCPs)—a complex group of unintended proteins derived from the expression system (commonly E. coli, CHO, or yeast). These HCPs may include secreted metabolic enzymes, stress-response proteins, membrane-associated fragments, as well as intracellular proteins released during cell lysis or death, all of which can be unintentionally carried through downstream processing and introduced into the final recombinant collagen product. These proteins may pose risks such as immunogenicity, product degradation, or functional interference. MtoZ Biolabs offers specialized Recombinant Collagen Host Cell Protein (HCP) Testing Services which support product safety, process optimization, and regulatory compliance.
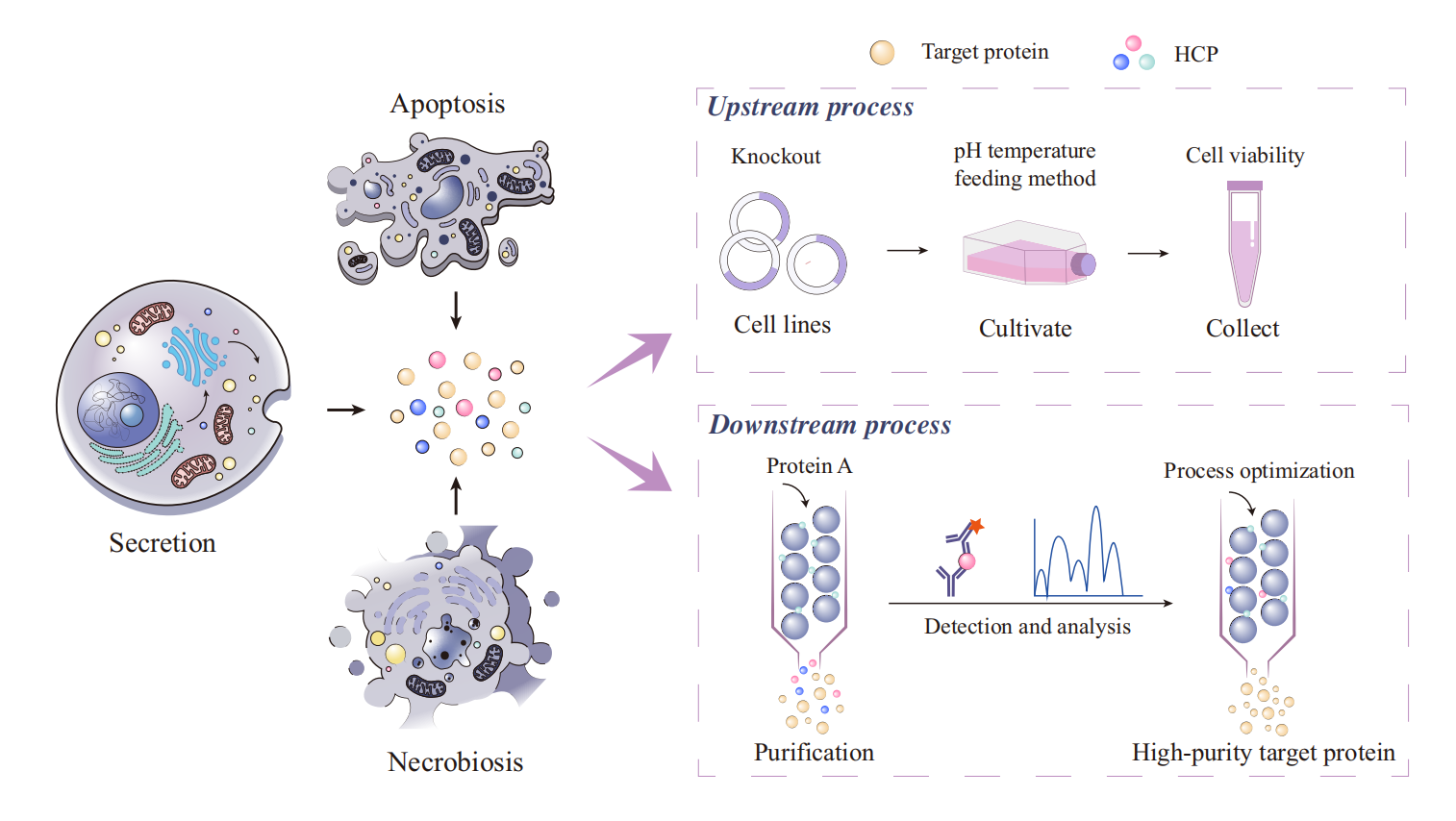
Figure 1. Source of Host Cell Proteins (HCPs) and Related Upstream and Downstream Processes
Service at MtoZ Biolabs
MtoZ Biolabs provides a comprehensive range of Recombinant Collagen Impurities Profile Service, including host cell protein (HCP) detection, heavy metals and trace elements analysis and more. MtoZ Biolabs' Recombinant Collagen Host Cell Protein (HCP) Testing Service employs a combination of enzyme-linked immunosorbent assay (ELISA) and mass spectrometry (MS)-based methods to detect and quantify residual HCPs in recombinant collagen products. This dual approach ensures both regulatory-recognized quantification and in-depth HCP profiling, allowing for accurate risk assessment and improved process control.
1. ELISA-Based HCP Quantification
ELISA is the industry standard for routine HCP quantification due to its high sensitivity, throughput, and regulatory acceptance. It uses antibodies capable of recognizing a broad range of host-derived proteins to achieve absolute quantification of total HCP content in recombinant collagen samples. Before applying the assay, antibody coverage validation is strongly recommended. Since commercial ELISA kits are developed using antigens from standard host cells, differences between the client’s expression system and the kit reference may limit detection. Verifying that the antibodies can recognize the full HCP spectrum is therefore critical.
To assess antibody coverage, we use Two-Dimensional Difference Gel Electrophoresis (2D-DIGE) to compare total host cell proteins with those detected by the ELISA antibodies. This allows us to visually and quantitatively assess how many of the expressed proteins are effectively recognized by the antibody pool, confirming the kit’s suitability. Once validated, the ELISA assay is used for accurate detection of residual HCPs in actual collagen product samples.
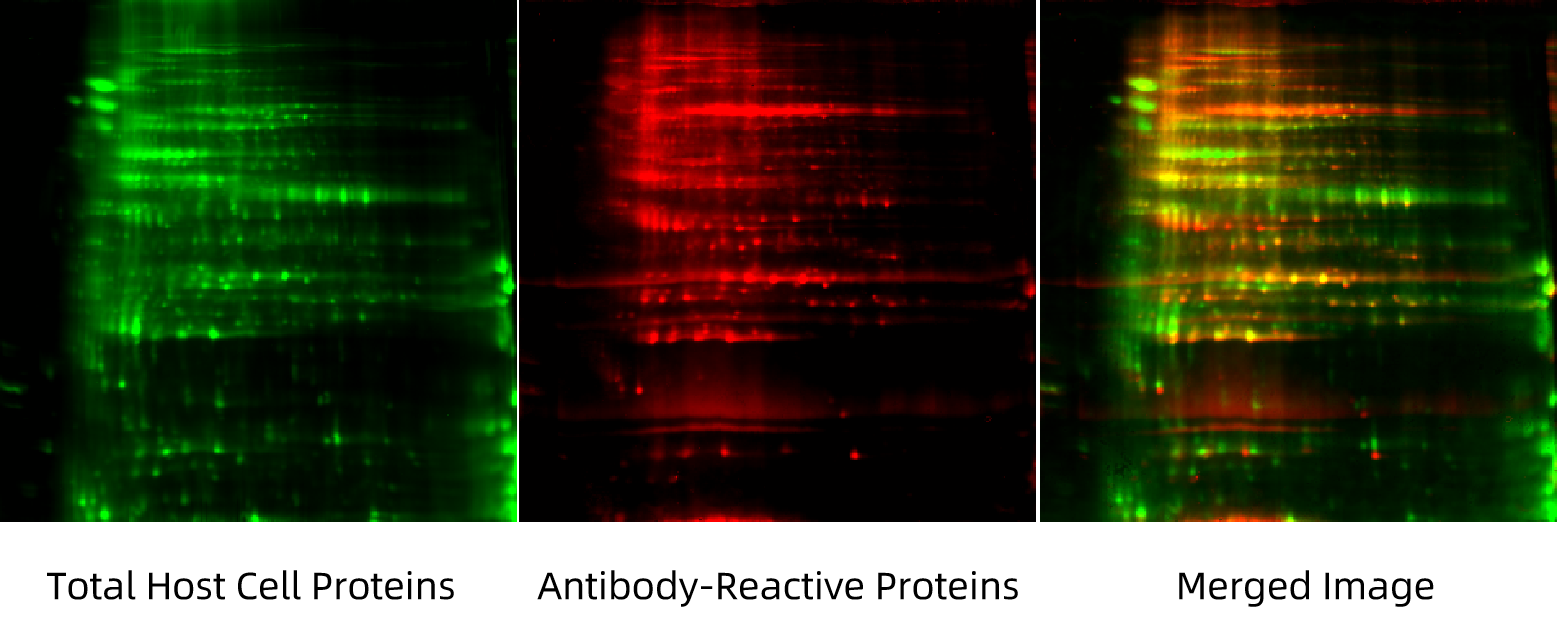
Figure 2. Antibody Coverage Assessment for HCP Detection Using 2D-DIGE
2. MS-Based HCP Identification and Profiling
To complement the ELISA results and provide a more comprehensive understanding of residual impurities, we also offer label-free LC-MS/MS-based HCP analysis. This technique enables the direct identification and relative quantification of individual HCP species remaining in purified recombinant collagen products.
Our high-resolution mass spectrometry workflow allows for:
💠Unbiased detection of HCPs not recognized by ELISA antibodies
💠Molecular identification of each residual protein, including potential immunogenic or proteolytic contaminants
💠Comparative profiling across different purification stages or production batches
💠Support for custom antibody development, if certain critical HCPs need to be specifically targeted in follow-up ELISA kits
Analysis Workflow
1. ELISA-Based HCP Quantification Workflow
1️⃣Sample Preparation: Dilute recombinant collagen samples to the appropriate concentration and buffer condition.
2️⃣Antibody Binding: Incubate samples with HCP-specific capture antibodies in microplate wells.
3️⃣Signal Detection: Add enzyme-conjugated secondary antibodies and substrate to generate a colorimetric signal.
4️⃣Data Acquisition: Measure absorbance and calculate HCP concentration using a standard curve.
5️⃣Result Reporting: Output HCP levels in ng/mg of total protein, along with detection range and assay controls.
2. LC-MS/MS-Based HCP Identification Workflow
1️⃣Protein Digestion: Denature collagen samples and enzymatically digest proteins (e.g., with trypsin).
2️⃣Peptide Cleanup: Purify digested peptides to remove salts and contaminants.
3️⃣LC-MS/MS Acquisition: Perform high-resolution mass spectrometry analysis to detect peptide ions.
4️⃣Database Search: Match MS/MS spectra to host cell protein databases for identification.
5️⃣Data Analysis and Reporting: Report identified HCP species, peptide counts, and relative abundance.
Service Advantages
1. Collagen-Focused Expertise
Extensive experience in detecting and characterizing impurities specific to recombinant collagen products.
2. Advanced Analysis Platform
Combined ELISA and LC-MS/MS approaches provide both quantitative accuracy and in-depth protein identification.
3. Custom Antibody Validation Support
Antibody coverage is assessed using 2D-DIGE to ensure ELISA reliability for your expression system.
4. Flexible Testing Options
Supports multiple host systems, production stages, and sample types with customizable testing plans.
5. End-to-End Analytical Capability
Integrated impurity testing including HCPs, residual DNA, heavy metals, and other critical quality attributes.
Sample Submission Suggestions
1. Sample Types
2. Storage Conditions
Store at –80 °C to maintain sample stability.
3. Shipping Conditions
Ship on dry ice with proper labeling and accompanying sample information.
*MtoZ Biolabs accepts samples from multiple expression systems and provides buffer exchange and pre-processing services upon request. Please contact us before shipping for any special requirements or pre-analysis consultation.
Deliverables
1. Overview of analytical methods and instrumentation
2. Raw data files
3. HCP quantification results
4. Mass spectra, chromatograms, and standard curves (as applicable)
5. Customized summary report suitable for internal review or regulatory submission
MtoZ Biolabs provides accurate, reliable, and flexible HCP analysis solutions tailored to your expression system, process needs, and regulatory goals. To learn more or request a quote, please contact us.
Related Services
Recombinant Collagen Impurities Profile Service
Recombinant Collagen Endotoxins Analysis Service
Recombinant Collagen Host Cell DNA Testing Service
Recombinant Collagen Heavy Metals & Trace Elements Analysis Service
How to order?







