Recombinant Collagen Endotoxins Analysis Service
Endotoxins, primarily lipopolysaccharides (LPS) originating from bacterial outer membranes, pose significant safety risks in recombinant collagen-based products. Contamination may occur during expression, purification, or packaging, and even trace levels of endotoxins can elicit strong immune responses, jeopardizing the clinical utility of biomedical products.
MtoZ Biolabs offers a dedicated Recombinant Collagen Endotoxins Analysis Service that employs the Limulus Amebocyte Lysate (LAL) assay to deliver high-sensitivity, quantitative detection across all production stages—from upstream intermediates to final drug substances. Our service ensures your recombinant collagen meets stringent biosafety standards for therapeutic, cosmetic, and tissue engineering applications.
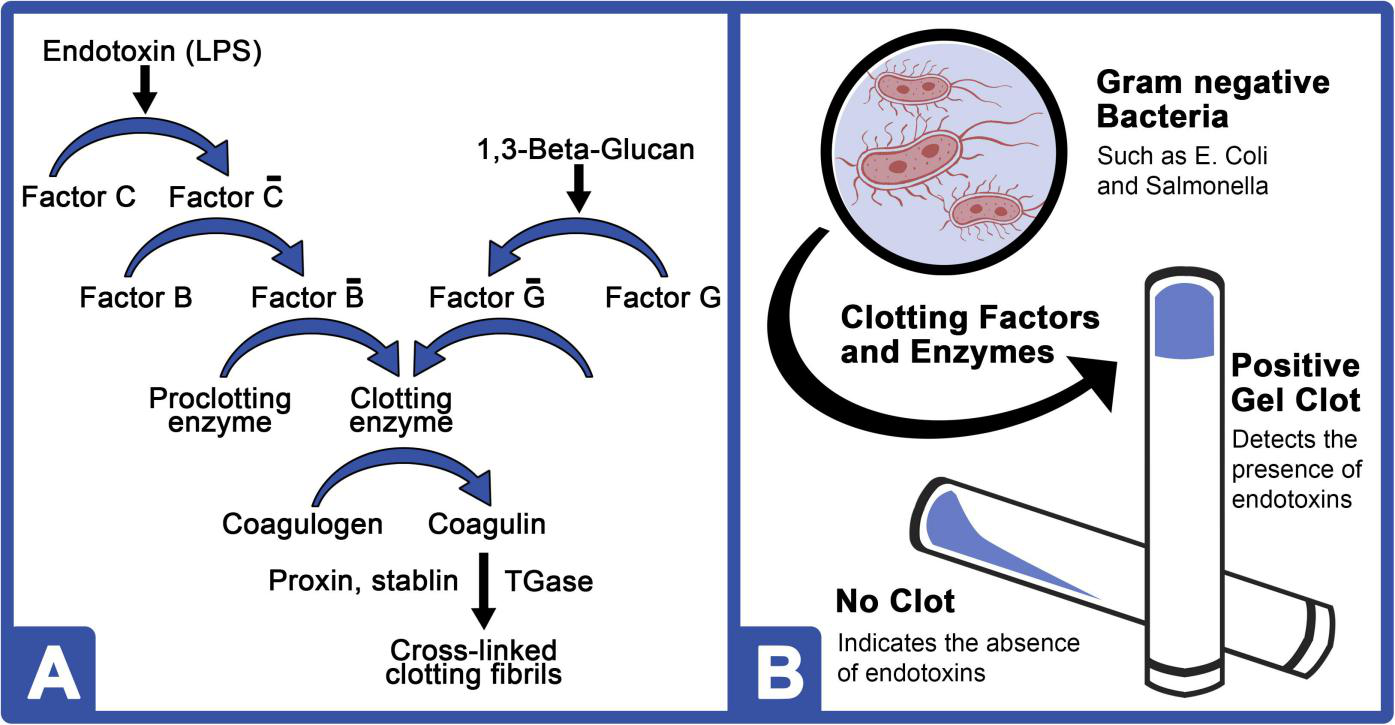
Tinker-Kulberg, R. et al. Front. Mar. Sci. 2020.
Figure 1. Schematic Diagram of Endotoxin Detection by LAL Gel Clot Method
Analysis Workflow
The Recombinant Collagen Endotoxins Analysis Service covers the entire workflow from sample preparation to final reporting, ensuring that each step meets stringent quality control standards.
1. Sample Receipt and Verification
Confirm sample ID, batch number, and testing requirements to ensure consistency between submitted material and analytical scope.
2. Sample Preparation
Conduct buffer exchange and dilution based on matrix type to optimize LAL compatibility and minimize assay interference.
3. Endotoxin Detection via LAL Assay
Perform quantitative endotoxin testing using gel-clot, chromogenic, or turbidimetric LAL-based formats, depending on sensitivity and sample type.
4. Data Analysis and Evaluation
Interpret assay results against relevant specifications; assess biosafety compliance and identify potential contamination sources.
5. Comprehensive Reporting
Deliver a detailed report including raw data, test method, quantified endotoxin levels, and expert interpretation with improvement recommendations if necessary.
Service Advantages
✅ Advanced Detection Platforms
Integration of gel-clot, kinetic chromogenic, and turbidimetric LAL assay formats ensures broad adaptability and methodological accuracy.
✅ High Sensitivity and Precision
Capable of detecting endotoxin levels as low as 1 EU/mL, enabling confident assessment of trace contaminants in complex collagen matrices.
✅ Custom Data Reporting and Consultation
Each client receives tailored reports with in-depth technical analysis, regulatory context, and process optimization advice.
✅ Broad Sample Compatibility
Suitable for purified collagen solutions, lyophilized powders, culture supernatants, and other bioprocess-related matrices.
Sample Submission Suggestions
|
Sample Type |
Recommended Amount |
Notes |
|
Collagen solution |
≥100 μL (≥1 mg/mL) |
Avoid freeze-thaw cycles; indicate purification stage and buffer conditions |
|
Lyophilized powder |
≥1 mg |
Keep sealed and desiccated; avoid moisture exposure |
Shipping & Storage Recommendations
Liquid Samples: Ship at 4 °C with cold packs; store at –20 °C upon arrival.
Lyophilized Samples: Ship on dry ice; store at room temperature in a dry, dark environment.
Note: We accept various sample types and offer optional pretreatment services. Contact our technical support team for specific guidance.
Applications
MtoZ Biolabs’ Recombinant Collagen Endotoxins Analysis Service supports safety evaluations in a wide range of industries and research settings:
1. Biopharmaceutical Quality Control: Verify endotoxin compliance across R&D and manufacturing phases to meet regulatory requirements.
2. Tissue Engineering and Regenerative Medicine: Ensure endotoxin-free scaffolds for implantation to minimize immune risks.
3. Cosmetic and Skincare Products: Assess collagen safety for dermal formulations targeting sensitive skin applications.
4. Drug Delivery Vehicles: Confirm absence of endotoxin contaminants in collagen-based delivery systems for enhanced therapeutic safety.
Deliverables
1. Quantitative endotoxin levels reported in EU/mL or EU/mg
2. Comprehensive analysis report including methodology, raw data, result interpretation, and technical notes
3. Sample pretreatment summary (if applicable)
4. Customizable report format available upon request to support regulatory submissions or internal documentation
Recombinant Collagen Endotoxins Analysis Service enables high-confidence quality assurance for your biomedical products. For service inquiries, pricing, or technical consultation, please contact MtoZ Biolabs today.
FAQ
Q1: How long does the endotoxin testing process take?
Testing typically requires 2–3 weeks, covering sample handling, preparation, assay execution, and data reporting. We aim to deliver timely, accurate results aligned with your production or regulatory timeline.
Q2: What is the principle of the LAL assay in endotoxin detection?
The LAL assay utilizes an enzyme cascade derived from horseshoe crab amebocytes. When exposed to endotoxins (LPS), this system triggers coagulation reactions measurable by gel clot formation, turbidity change, or chromogenic signal, providing a sensitive and specific detection method.
Related Services
How to order?







