Recombinant Collagen Kanamycin Residue Testing Service
- We accepts a variety of sample types, including purified recombinant collagen, lyophilized powders, and more.
- Samples should be shipped at low temperatures and protected from repeated freeze-thaw cycles.
- Lyophilized powders should be stored in a dry, sealed container and protected from moisture.
Recombinant Collagen Kanamycin Residue Testing Service is a specialized analytical solution designed to detect and quantify trace levels of kanamycin in recombinant collagen products, ensuring safety and regulatory compliance. Recombinant collagen, synthesized through microbial or eukaryotic expression systems, is a widely used biomaterial in regenerative medicine, wound healing, cosmetics, and pharmaceutical formulations. During large-scale upstream production, especially in bacterial expression systems such as E. coli, antibiotics like kanamycin are commonly used for plasmid selection and maintenance of genetic constructs. Kanamycin is an aminoglycoside antibiotic known for its nephrotoxicity and ototoxicity in humans. Even trace amounts of residual kanamycin in injectable, implantable, or topical collagen-based products can lead to adverse immunological or toxicological responses, especially when used in sensitive populations or for long-term applications. In response to these safety concerns, international regulatory authorities have established strict limits on residual antibiotic content in biopharmaceuticals and biomaterials.
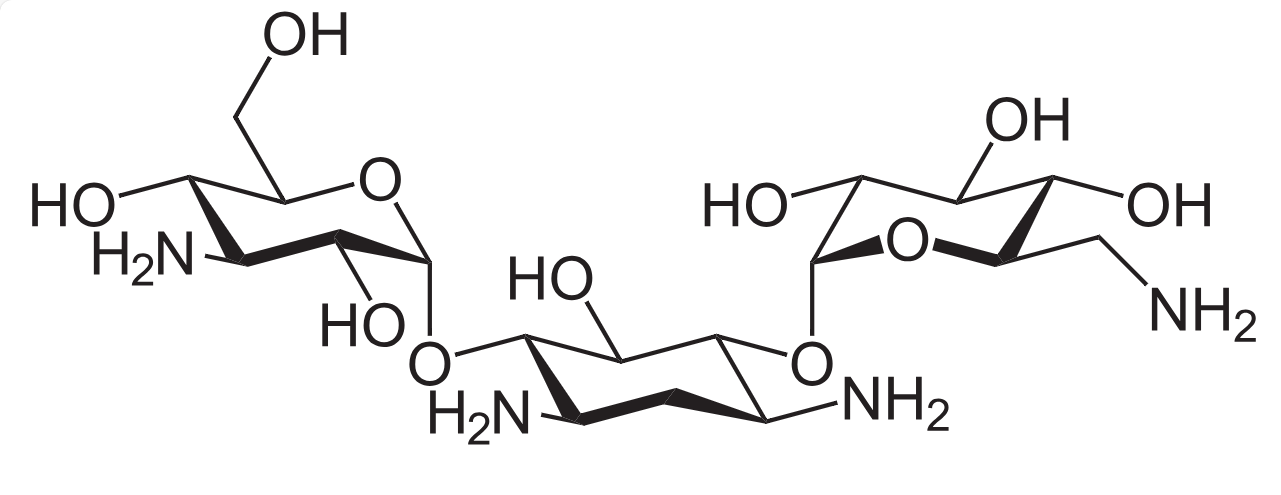
Figure 1. Chemical Structure of Kanamycin
Service at MtoZ Biolabs
To meet stringent regulatory requirements and ensure product safety, MtoZ Biolabs offers a Recombinant Collagen Kanamycin Residue Testing Service that delivers precise and reliable quantification of residual kanamycin in complex protein matrices. The Recombinant Collagen Kanamycin Residue Testing Service integrates multiple validated analytical techniques—including high-performance liquid chromatography (HPLC), ultra-sensitive liquid chromatography–mass spectrometry (LC-MS/MS), and enzyme-linked immunosorbent assay (ELISA)—to provide flexible, high-accuracy detection tailored to different sample types and sensitivity needs.
Analysis Workflow
ELISA-Based Workflow for Kanamycin Residue Detection:
1. Sample Preparation
Collagen samples are diluted or pretreated to release kanamycin into solution.
2. Reagent Addition
Samples, standards, and enzyme-labeled reagents are added to a microplate coated with kanamycin-specific antibodies or conjugates.
3. Incubation and Washing
The plate is incubated to allow binding, followed by washing to remove unbound substances.
4. Color Development
A substrate is added to produce a color reaction inversely proportional to kanamycin concentration.
5. Result Reading
Absorbance is measured with a microplate reader, and concentrations are determined by comparison to a standard curve.
Service Advantages
☑️Matrix-Compatible Sample Handling: Optimized workflows tailored for recombinant collagen ensure accurate detection in complex protein backgrounds.
☑️Multiple Detection Options: Offers ELISA for rapid screening and LC-MS/MS for high-sensitivity quantification, meeting diverse project needs.
☑️Fast Turnaround and High Throughput: Efficient testing pipeline supports both routine QC and large-scale batch analysis.
☑️Experienced Technical Team: Our scientists provide expert guidance on sample preparation, method selection, and data interpretation.
☑️Reliable and Reproducible Results: Strict quality control ensures consistency and confidence in every dataset delivered.
Applications
Our Kanamycin Residue Testing Service supports a wide range of applications in recombinant collagen development and quality control:
1. Biomedical Product Safety Evaluation: Ensures residual kanamycin levels in injectable or implantable collagen products meet regulatory safety thresholds.
2. Cosmetic Ingredient Compliance: Verifies antibiotic-free status of collagen used in topical formulations, aligning with cosmetic safety regulations in global markets.
3. Regulatory Documentation and Batch Release: Supports product registration, quality control documentation, and batch release testing by providing validated kanamycin residue data in compliance with relevant regulatory standards.
4. Process Optimization and Risk Assessment: Helps manufacturers evaluate antibiotic carryover from upstream processes and improve purification efficiency.
5. Academic and Translational Research: Supports preclinical studies requiring antibiotic-free biomaterials to avoid immunological interference or cytotoxic effects.
Sample Submission Suggestions
If your samples require special handling, please contact us. We will provide customized submission instructions and pretreatment recommendations based on the sample type and analytical requirements.
Deliverables
1. Kanamycin concentration results
2. Standard curve and absorbance data
3. Summary of assay method and kit used
4. Quality control information
5. Final report with result interpretation
How to order?







