Pharmaceutical Thermal Stability Analysis Service
Pharmaceutical thermal stability analysis is an essential analytical approach used to evaluate the physical and chemical stability of drug substances or formulations under varying temperature conditions. Its core principle involves utilizing multiple thermal analysis techniques to systematically assess changes in the physicochemical properties of drugs and excipients upon heating, aiming to reveal decomposition behaviors, phase transitions, reaction characteristics, and thermal stability. This service employs highly sensitive thermal analysis platforms and integrates multidimensional data—including mass change, heat flow response, and structural alterations—to comprehensively reflect performance changes and potential risks of drugs during thermal exposure.
The pharmaceutical thermal stability analysis service is widely applied in drug development, quality control, and process optimization. It can be used to investigate the storage stability of candidate drugs, evaluate the compatibility of excipients with active ingredients, guide the setting of formulation process parameters, and assist in selecting suitable packaging materials. This plays a critical role in ensuring the safety and efficacy of pharmaceuticals throughout manufacturing, transportation, and usage.
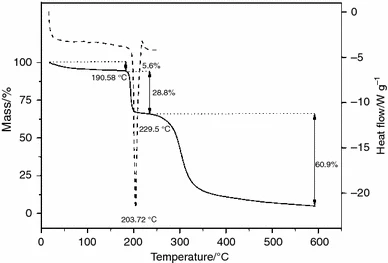
Júlio, T A. et al. Journal of Thermal Analysis and Calorimetry, 2012.
Figure 1. DSC and TG curves of sildenafil citrate (SC).
Services at MtoZ Biolabs
Based on a variety of thermal analysis platforms, MtoZ Biolabs offers the pharmaceutical thermal stability analysis service to evaluate the thermal stability of research samples under different conditions. This service enables the precise determination of key thermal parameters, including melting point, glass transition temperature (Tg), decomposition temperature, and weight loss rate. These data comprehensively characterize the physical changes and degradation behavior of pharmaceutical substances during heating, providing robust support for formulation screening, storage condition design, manufacturing process control, and quality stability studies. The thermal analysis methods employed include, but are not limited to, the following:
1. GPC (Gel Permeation Chromatography)
Used to monitor molecular weight changes of polymeric drugs or excipients during high-temperature storage or processing, evaluating thermal degradation or polymerization behavior.
2. TPD/TPR/TPO (Temperature-Programmed Desorption/Reduction/Oxidation)
Analyzes gas-phase reaction behavior on the surface of drug carriers and excipients under thermal conditions, useful for studying the impact of heat treatment on drug system stability.
3. TGA (Thermogravimetric Analysis)
Evaluates the mass change of active pharmaceutical ingredients, intermediates, and excipients under heating conditions to determine decomposition temperature, weight loss characteristics, and thermal stability limits.
4. TGA-DSC (Thermogravimetric Analysis - Differential Scanning Calorimetry)
Simultaneously acquires thermogravimetric and heat flow data to reveal thermal behaviors such as melting, phase transitions, and decomposition, supporting formulation stability evaluation and impurity tracing.
5. TG-IR-GC/MS (Thermogravimetric Analysis–Infrared Spectroscopy–Gas Chromatography/Mass Spectrometry)
Identifies the molecular structure of degradation products during heating, analyzing the sources of volatile impurities and thermal decomposition pathways.
6. LFA (Laser Flash Analysis)
Measures the thermal diffusivity and thermal conductivity of solid pharmaceutical formulations to assess heat transfer performance, supporting the design of stable storage and transportation conditions.
Analysis Workflow
1. Sample Preparation
After the client submits the pharmaceutical sample, technicians confirm sample information, physical state, and relevant background details, followed by necessary pretreatment to ensure compatibility with subsequent testing conditions.
2. Method Setup
Based on the drug properties and analysis objectives, appropriate thermal analysis methods are selected. Parameters such as temperature range, heating rate, and atmospheric conditions are set to ensure scientific and reasonable experimental design.
3. Data Acquisition
Under the defined conditions, the sample undergoes programmed heating. Multi-dimensional data such as mass change, heat flow, and gas release are collected in real time, capturing the full thermal behavior of the sample.
4. Result Analysis
The collected data is professionally interpreted to extract key parameters and generate a standardized analysis report, supporting clients in stability evaluation and process optimization.
Sample Submission Suggestions
1. Sample Type
It is recommended to provide purified solid or powder pharmaceutical samples. For samples in special forms (e.g., liquids, lyophilized materials), please specify in advance to allow selection of appropriate testing protocols.
2. Storage and Transportation
Samples should be sealed in dry, clean containers to avoid moisture and heat exposure. Standard samples can be shipped at room temperature; for temperature- or humidity-sensitive samples, cold-chain transportation with desiccants is recommended.
3. Additional Information
Please indicate the nature of the sample and intended testing objectives to ensure proper workflow planning and enhance the accuracy and comparability of the data.
Service Advantages
1. Integrated Technologies
Combines multiple thermal analysis techniques, including thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), laser flash analysis (LFA), and chemisorption, to comprehensively characterize drug thermal behavior under varying temperature conditions.
2. High-Sensitivity Detection
Utilizes high-performance instruments such as TGA-DSC and LFA to accurately capture mass changes and thermal effects of pharmaceutical samples, ensuring result stability and reproducibility.
3. One-Time-Charge
Our pricing is transparent, no hidden fees or additional costs.
4. One-Stop Service
Delivers end-to-end support—from sample preparation and method selection to experiment execution and data interpretation—saving clients time and enhancing analytical efficiency and user experience.
Applications
1. Formulation Screening
Thermal behavior analysis supports the selection of excipients and carriers with favorable thermal stability, optimizing pharmaceutical formulations and improving final product quality.
2. Process Optimization
Pharmaceutical thermal stability analysis service helps evaluate the thermal response of APIs and formulations under different temperature conditions, guiding the setting of process parameters for drying, sterilization, and tablet compression to avoid thermal degradation risks.
3. Packaging Compatibility
Analyzes the thermal compatibility between drugs and packaging materials during the packaging process, ensuring proper material selection and maintaining product stability during storage and transport.
4. Storage and Transport Evaluation
Pharmaceutical thermal stability analysis service is used to study thermal stability changes during long-term storage or transportation, providing a scientific basis for temperature control strategies and shelf-life determination.
FAQ
Q1: Does the Analysis Offer Good Repeatability and Accuracy?
A1: Yes. MtoZ Biolabs is equipped with high-precision thermal analysis instruments and follows standardized testing procedures to ensure highly repeatable and reliable results.
Q2: Can the Testing Plan Be Customized for Specific Research Needs?
A2: Yes. We support flexible combinations of thermal analysis techniques tailored to research goals such as formulation screening, storage stability, and process compatibility, delivering fully customized solutions.
Q3: Are Raw Data and Thermal Plots Provided?
A3: Yes. Each report includes raw data, thermal curve plots, key parameters (such as onset decomposition temperature, peak temperature, and weight loss percentage), along with technical interpretation.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Data Analysis, Preprocessing, and Estimation
4. Bioinformatics Analysis
5. Raw Data Files
Related Services
Temperature Programmed Analytical (TPD/TPR/TPO) Service
Thermogravimetric Analysis (TGA) Service
Thermogravimetric Analysis - Differential Scanning Calorimetry (TGA-DSC) Analytical Service
How to order?







