Fc-Fusion Protein Drug Glycosylation Characterization Service
Fc-fusion proteins represent a rapidly expanding class of biotherapeutics, designed to combine the functional domain of a biologically active protein with the constant region (Fc) of an immunoglobulin. This molecular configuration extends the serum half-life of the therapeutic protein through neonatal Fc receptor (FcRn)-mediated recycling, in which the Fc domain binds FcRn within endosomes and is protected from lysosomal degradation before being returned to the circulation. It also enhances stability, solubility, and manufacturability. Fc-fusion drugs are now widely applied in the treatment of autoimmune diseases, cancers, and rare genetic disorders, and their market share continues to grow.
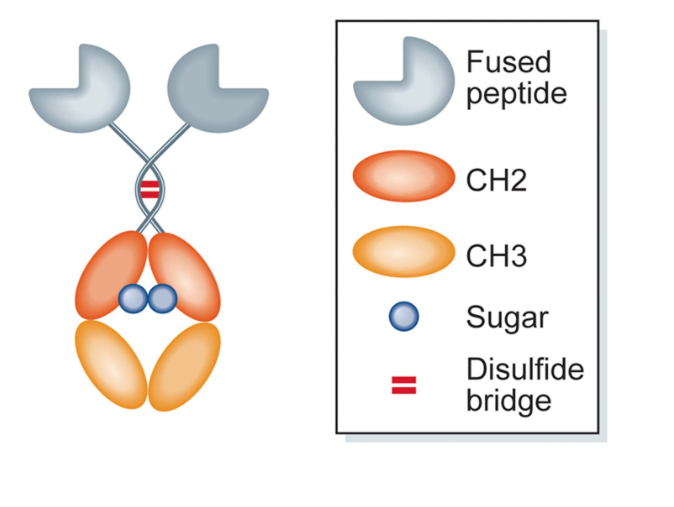
Figure 1. The Structure of a Prototypic IgG Fc‐Fusion
The structural complexity of Fc-fusion proteins presents unique analytical challenges. One of the most critical post-translational modifications affecting these molecules is glycosylation. Variations in glycan composition, branching, sialylation, and fucosylation between the Fc and fusion regions can impact pharmacokinetics, receptor interactions, immunogenicity, and overall biological activity. In particular, Fc glycosylation patterns modulate effector functions such as antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and antibody-dependent cellular phagocytosis (ADCP), which play a vital role in therapeutic mechanisms of action and clinical performance. Fc-fusion protein drug glycosylation characterization refers to the in-depth analytical evaluation of glycan structures, site-specific glycosylation occupancy, and glycoform distribution on the full-length fusion protein. This analysis is essential for defining critical quality attributes, ensuring batch-to-batch consistency, and meeting stringent regulatory expectations.
Service at MtoZ Biolabs
To support the successful development, validation, and release of Fc-fusion biologics, MtoZ Biolabs offers a comprehensive Fc-Fusion Protein Drug Glycosylation Characterization Service tailored to their specific structural and functional features. Our platform integrates orthogonal analytical technologies to comprehensively assess glycan composition, structure, site occupancy, and functional relevance.
We employ advanced methodologies such as liquid chromatography–mass spectrometry (LC-MS), high-performance liquid chromatography (HPLC), hydrophilic interaction liquid chromatography coupled with ultra-performance liquid chromatography (HILIC-UPLC), matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS), capillary electrophoresis with laser-induced fluorescence (CE-LIF), and gas chromatography–mass spectrometry (GC-MS). These tools allow precise glycan detection, isomer resolution, and quantitative analysis at multiple structural levels. With support for released glycan profiling, glycopeptide mapping, and intact glycoprotein analysis, our services are designed to provide complete insights into the glycosylation landscape of both the Fc and fusion domains.
Why Choose MtoZ Biolabs?
☑️Advanced Analysis Platform: MtoZ Biolabs established an advanced Fc-Fusion Protein Drug Glycosylation Characterization Service platform, guaranteeing reliable, fast, and highly accurate analysis service.
☑️High-Data-Quality: Deep data coverage with strict data quality control. AI-powered bioinformatics platform integrates all protein drug glycosylation characterization data, providing clients with a comprehensive data report.
☑️Specialized Expertise in Fc-Fusion Proteins: Deep experience in addressing the unique glycosylation complexity of Fc-fusion drugs.
☑️Customizable Solutions: Tailored analytical strategies to fit different development stages, from early discovery to commercial manufacturing.
☑️One-Time-Charge: Our pricing is transparent, no hidden fees or additional costs.
Applications
Fc-fusion protein drug glycosylation characterization plays a vital role across the biopharmaceutical development spectrum. MtoZ Biolabs supports the following applications:
1. Cell Line Screening
Evaluate glycan profiles to select expression systems that produce therapeutically favorable glycoforms.
2. Process and Formulation Development
Monitor glycosylation changes during upstream and downstream process adjustments or formulation optimization.
3. Biosimilarity and Comparability Studies
Demonstrate structural equivalence between biosimilars and reference products by comparing site-specific glycosylation patterns.
4. Quality Control
Assess batch-to-batch consistency by analyzing glycan profiles, structural uniformity, and potential deviations from defined specifications.
5. Stability Studies
Detect glycan degradation, desialylation, or aggregation under thermal, pH, or oxidative stress conditions.
6. Immunogenicity Risk Assessment
Identify non-human glycan epitopes such as N-glycolylneuraminic acid or alpha-gal that may induce adverse immune responses.
7. Mechanistic and Functional Studies
Investigate how specific glycan structures impact Fc receptor binding, biological half-life, or bioactivity of the fusion component.
Sample Submission Suggestions
1. Sample Type
Purified Fc-fusion proteins expressed in mammalian systems (e.g., CHO, HEK293)
2. Buffer Conditions
Avoid buffers containing glycerol, SDS, EDTA, or high salt concentrations
3. Storage and Shipping
Store samples at –20°C or lower. Ship on dry ice with complete sample information, including concentration, buffer composition, and sample origin.
4. Additional Documentation
If available, please provide production method details, or specific testing goals to support customized workflow design.
*Note: If you require additional guidance, please contact us for personalized support before sample preparation.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Total Ion Chromatogram & Quality Control Assessment (project-dependent)
4. Data Analysis, Preprocessing, and Estimation (project-dependent)
5. Bioinformatics Analysis
6. Raw Data Files
Related Services
Antibody Drug Glycosylation Characterization Service
Protein Drug Glycosylation Characterization Service
Glycoprotein Profiling Service
Glycoprotein Structure Analysis Service
How to order?







