Protein Drug Glycosylation Characterization Service
- Protein-based drugs such as cytokines, purified monoclonal antibodies, antibody-drug conjugates, Fc-fusion proteins, or other therapeutic glycoproteins
- Preferred: PBS, Tris, or ammonium acetate buffers without interfering substances
- Avoid: EDTA, SDS, glycerol, DTT, or high salt concentrations
- Samples should be stored at -80℃ and shipped with dry ice
Protein-based drugs, including therapeutic enzymes, cytokines, hormones, fusion proteins, and engineered scaffolds, have become integral to modern biopharmaceuticals. These biologics offer highly targeted mechanisms of action and diverse treatment applications across oncology, metabolic diseases, autoimmune disorders, and rare genetic conditions. Their clinical efficacy and safety are closely tied to their structural properties, with glycosylation being one of the most critical post-translational modifications. Glycosylation refers to the covalent attachment of carbohydrate chains (glycans) to specific amino acid residues on a protein. This modification affects a wide range of biological attributes, including solubility, folding, stability, immunogenicity, biological half-life, receptor binding, and downstream signaling. Both N-linked glycosylation and O-linked glycosylation contribute to the overall molecular heterogeneity and functional complexity of protein drugs.

Figure 1. Examples of Glycan Structures That can be Found in Recombinant Monoclonal Antibodies, with Their Impact on Pharmacokinetics, Safety and Efficacy
Protein drug glycosylation characterization is the systematic analytical evaluation of glycan structures, site occupancy, and glycoform distribution on therapeutic proteins. Given its direct impact on drug safety and performance, many regulatory authorities classify glycosylation patterns as critical quality attributes. A thorough glycosylation analysis enables manufacturers to ensure batch-to-batch consistency, monitor process- or storage-induced variations, and identify potential risks associated with glycan heterogeneity. It also supports comparability assessments, formulation optimization, and regulatory submissions across all phases of drug development.
Service at MtoZ Biolabs
MtoZ Biolabs offers comprehensive Protein Drug Glycosylation Characterization Services to support biopharmaceutical research, development, and quality control. Our services are designed to help clients accurately profile glycosylation patterns, identify critical quality attributes, and meet regulatory expectations throughout the product lifecycle.
To meet the diverse analytical needs of glycan characterization, MtoZ Biolabs integrates a wide range of cutting-edge techniques, including high-performance liquid chromatography (HPLC), liquid chromatography–mass spectrometry (LC-MS), gas chromatography–mass spectrometry (GC-MS), matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS), and capillary electrophoresis (CE). By combining these orthogonal methods, we ensure high sensitivity, structural resolution, and quantitative reliability across various glycoforms and protein formats.
Our glycosylation characterization services include:
💠Monosaccharide Identification and Quantification
Analysis of core and terminal monosaccharide residues, including hexoses, N-acetylhexosamines, mannose, galactose, fucose, and others.
💠Sialic Acid Identification and Quantification
Sensitive detection and quantification of total and individual sialic acid species (e.g., N-acetylneuraminic acid, N-glycolylneuraminic acid).
💠Oligosaccharide Profiling
Structural analysis and relative quantification of released N- and O-linked glycans.
💠Glycosylation Site Mapping
Site-specific determination of glycan occupancy using proteolytic digestion and tandem mass spectrometry.
💠Site Occupation Analysis
Quantitative evaluation of glycan occupancy rates at each glycosylation site to assess structural consistency and modification efficiency.
💠Glycan Structural Elucidation
Detailed determination of glycan composition, branching, linkage types, and terminal modifications through exoglycosidase digestion and advanced MS/MS analysis.
Analysis Workflow
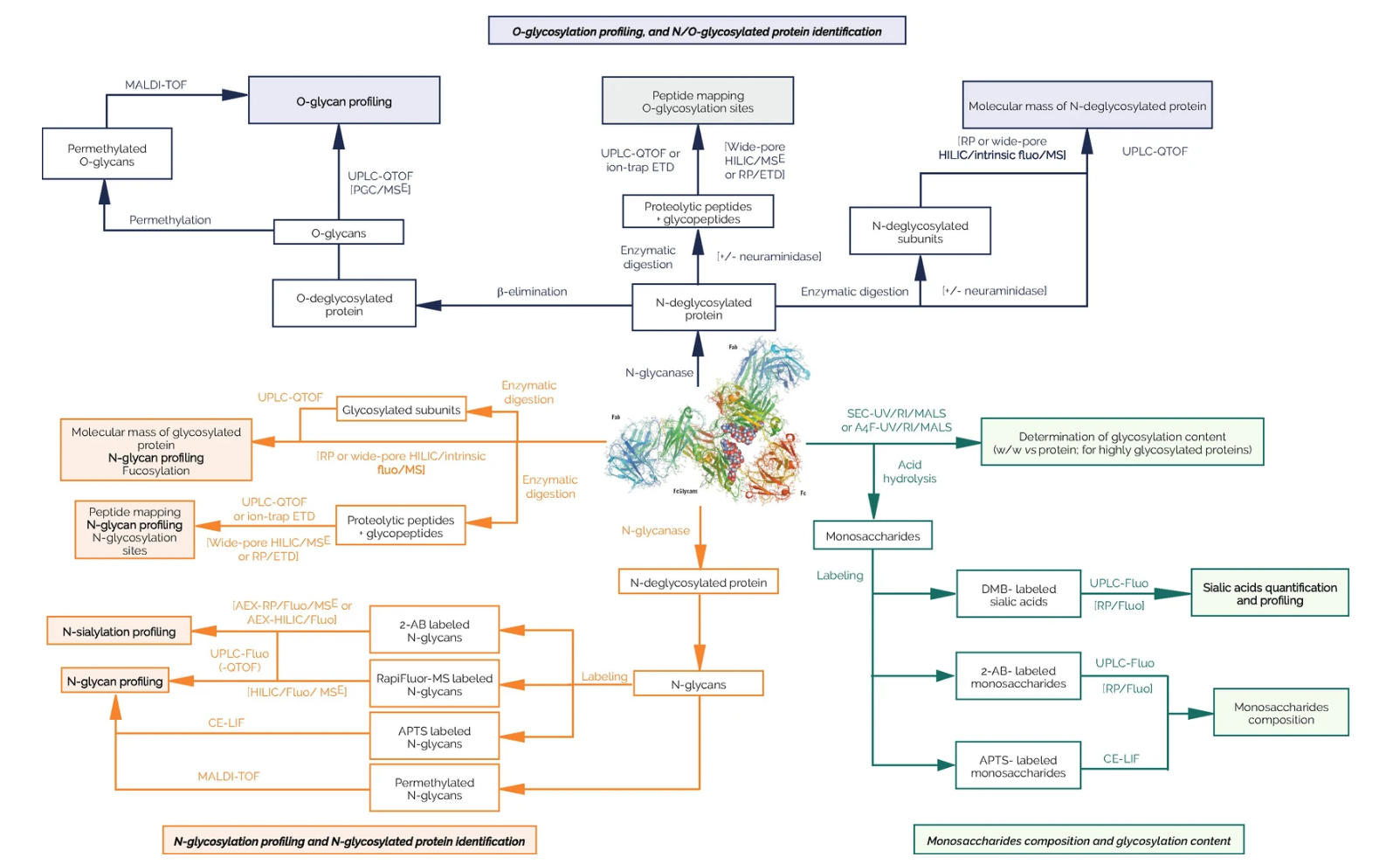
Figure 2. Analytical Workflows That can be used for the Characterization of Therapeutic Glycoproteins
Service Advantages
✔️Advanced Analysis Platform
MtoZ Biolabs established an advanced Protein Drug Glycosylation Characterization Service platform, guaranteeing reliable, fast, and highly accurate analysis service.
✔️High-Data-Quality
Deep data coverage with strict data quality control. AI-powered bioinformatics platform integrates all protein drug glycosylation characterization data, providing clients with a comprehensive data report.
✔️Comprehensive Service Scope
From released glycan analysis to intact glycoprotein mass profiling, our portfolio covers the full spectrum of glycosylation characterization.
✔️Customized Solutions
We tailor our workflow based on different protein types, production system, and client objectives.
✔️One-Time-Charge
Our pricing is transparent, no hidden fees or additional costs.
Applications
Our Protein Drug Glycosylation Characterization Service supports a wide range of applications in biopharmaceutical research and development:
1. Clone and cell line selection: Identify expression systems that produce desired glycosylation patterns (e.g., reduced fucosylation or increased sialylation).
2. Process development and scale-up: Monitor changes in glycosylation during upstream and downstream process optimization.
3. Quality control and release testing: Ensure batch-to-batch consistency by comparing glycan profiles across production lots.
4. Comparability studies for biosimilars and biobetters: Demonstrate structural equivalence or improvements in glycosylation relative to reference products.
5. Stability and formulation studies: Assess glycan degradation, de-sialylation, or aggregation under stress conditions.
6. Immunogenicity risk assessment: Detect non-human or unusual glycans that may trigger unwanted immune responses.
7. Mechanistic studies: Correlate glycan structure with pharmacodynamic or receptor binding activity.
Sample Submission Suggestions
1. Sample Types
We accept various sample types, including but not limited to:
2. Buffer Requirements
3. Storage and Shipping
*Note: If you have special sample types or require additional guidance, please contact our technical team for personalized support before sample preparation.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Total Ion Chromatogram & Quality Control Assessment (project-dependent)
4. Data Analysis, Preprocessing, and Estimation (project-dependent)
5. Bioinformatics Analysis
6. Raw Data Files
Contact MtoZ Biolabs today to discuss your protein drug glycosylation analysis needs and receive a customized consultation and quotation.
Related Services
Glycoprotein Profiling Service
Glycoprotein Structure Analysis Service
How to order?







