Drug Glycosylation Characterization Service
Drug glycosylation characterization refers to the comprehensive analysis of carbohydrate modifications (glycans) attached to therapeutic drugs, particularly biologics such as monoclonal antibodies, Fc-fusion proteins, therapeutic enzymes, and glycoengineered molecules. Glycosylation is one of the most critical post-translational modifications (PTMs) in biopharmaceuticals, significantly influencing drug stability, solubility, efficacy, immunogenicity, and pharmacokinetics.
In therapeutic proteins, glycosylation affects key biological mechanisms, including antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and antibody-dependent cellular phagocytosis (ADCP). Terminal sugars such as sialic acids can extend serum half-life by modulating clearance pathways, while fucosylation and galactosylation influence effector functions. Because glycosylation patterns are determined by cellular machinery and manufacturing conditions, they can vary between production batches, cell lines, or process changes. Regulatory agencies classify glycosylation profiles as critical quality attributes, requiring robust and reproducible characterization. Drug glycosylation characterization is therefore essential for drug discovery, process development, quality control, comparability assessments, and regulatory submissions. It provides detailed information on glycan composition, site occupancy, linkage type, and heterogeneity, enabling manufacturers and researchers to ensure consistent product quality and therapeutic performance.
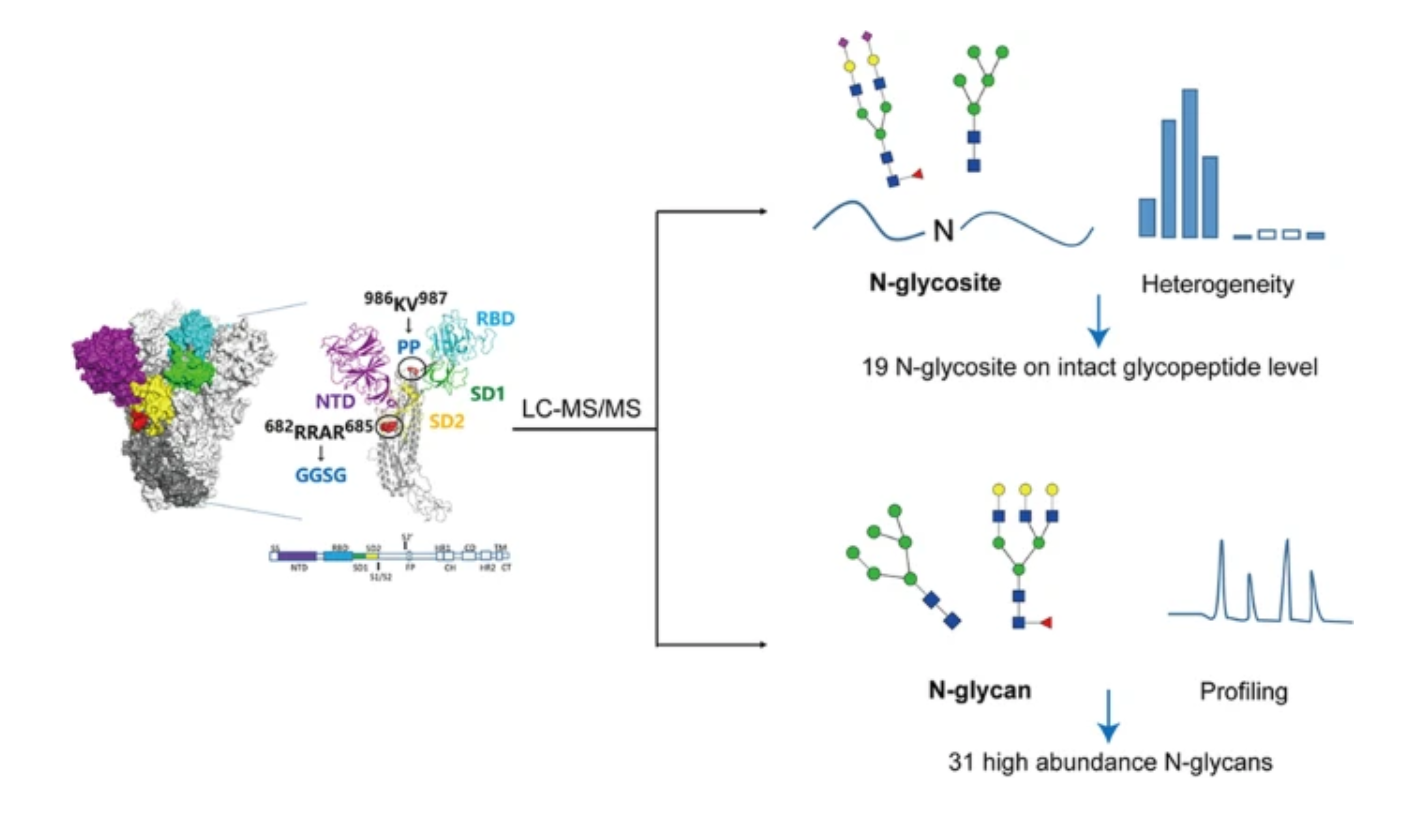
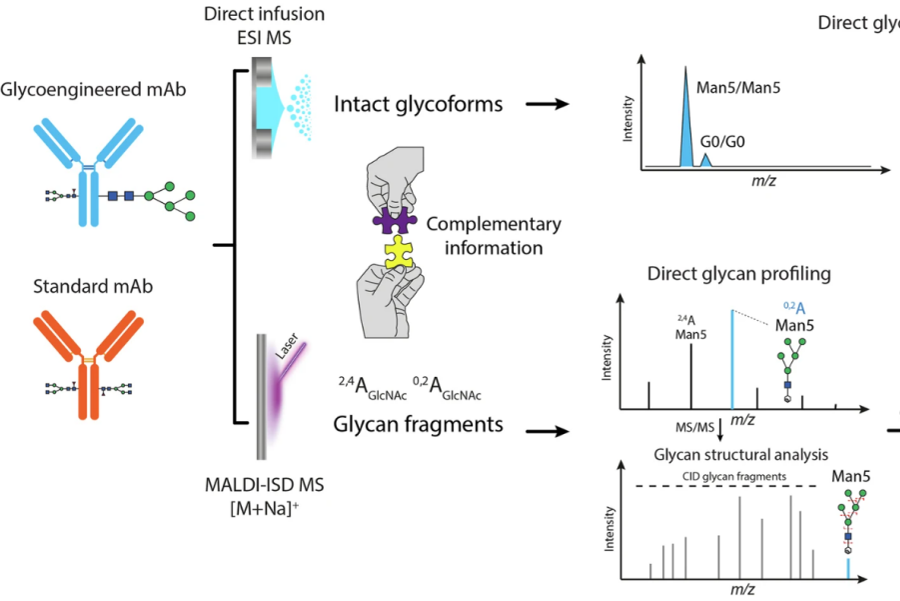
Huang, J. et al. Anal Bioanal Chem. 2023.
Figure 1. In-Depth Characterization of Protein N-Glycosylation for a COVID-19 Variant-Design Vaccine Spike Protein
Service at MtoZ Biolabs
MtoZ Biolabs provides end-to-end Drug Glycosylation Characterization Services that combine advanced analytical platforms with deep glycoproteomics expertise. Our solutions are designed to capture both qualitative and quantitative glycosylation data, from released glycan profiling to site-specific glycopeptide mapping and intact glycoprotein analysis.
Analysis Workflow

Delobel, A. Methods in Molecular Biology. 2021.
Figure 2. Analytical Workflows That can be Used for the Characterization of Therapeutic Glycoproteins
Service Advantages
✔️Multi-Level Glycosylation Analysis
Comprehensive profiling from released glycans to site-specific glycopeptides and intact glycoprotein analysis, enabling a full understanding of glycosylation patterns and functional implications.
✔️State-of-the-Art Analytical Platforms
Advanced instrumentation including high-resolution LC-MS/MS, HILIC-UPLC, CE-LIF, and MALDI-TOF MS ensures high sensitivity, structural accuracy, and reproducibility in glycosylation characterization.
✔️Expert Scientific Team
A dedicated team with extensive expertise in glycoproteomics and regulatory-compliant biopharmaceutical analytics, capable of handling complex glycosylation challenges.
✔️Customizable Solutions
Tailored workflows to meet diverse project needs, from early-stage research to commercial manufacturing.
✔️One-Time-Charge
Our pricing is transparent, no hidden fees or additional costs.
Applications
1. Biopharmaceutical development, including optimization of therapeutic glycoproteins for stability, efficacy, and safety
2. Process monitoring and quality control to ensure batch-to-batch glycosylation consistency
3. Biosimilarity and comparability assessments for regulatory submissions
4. Disease-related glycoprotein research for biomarker discovery and functional analysis
5. Evaluation of manufacturing changes on glycosylation profiles
Sample Submission Suggestions
To ensure optimal results, please follow these recommendations:
🔸Sample Types: Purified therapeutic proteins, antibodies, Fc-fusion proteins, or other glycoprotein drugs
🔸Minimum Amount: 50–100 μg for purified proteins; larger amounts may be required for complex analyses
🔸Concentration: 0.5–2 mg/mL preferred
🔸Buffer Requirements: Avoid high concentrations of salts, detergents, or glycerol
🔸Storage: Store at –80°C; ship on dry ice
*Note: If you have special sample types or require additional guidance, please contact us for personalized support before sample preparation.
Deliverables
1. Comprehensive Experimental Summary
2. Detailed Glycosylation Profiles
3. Structural Elucidation Data
4. Quantitative Analysis
5. Visual Outputs (chromatograms, spectra, and annotated glycan structures)
6. Raw Data Files
7. Interpretation Report with functional relevance and recommendations
Related Services
Glycoprotein Profiling Service
Glycoprotein Structure Analysis Service
How to order?