Antibody Drug Glycosylation Characterization Service
- Purified monoclonal antibodies, antibody-drug conjugates, Fc-fusion proteins, or other glycoproteins
- Preferred: PBS, Tris, or ammonium acetate buffers without interfering substances
- Avoid: EDTA, SDS, glycerol, DTT, or high salt concentrations
- Samples should be stored at -80℃ and shipped with dry ice
Antibody-based drugs, including monoclonal antibodies (mAbs), antibody-drug conjugates (ADCs), and bispecific antibodies, have emerged as one of the most prominent classes of biotherapeutics. These biologics are characterized not only by their specificity and potency but also by their structural complexity, with glycosylation being a critical determinant of their function, pharmacokinetics, immunogenicity, and manufacturability. Glycosylation refers to the enzymatic attachment of oligosaccharide chains to specific amino acid residues, most commonly the asparagine (N-linked) or serine/threonine (O-linked) residues of proteins. For antibodies, glycosylation predominantly occurs at the Fc domain and plays a pivotal role in modulating antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and Fc receptor binding. Additionally, glycan profiles influence solubility, stability, serum half-life, and antigen binding of therapeutic antibodies.
Antibody drug glycosylation characterization refers to the comprehensive analysis of glycan structures, compositions, and occupancy on therapeutic antibodies. This characterization enables researchers and developers to assess glycan heterogeneity, identify critical quality attributes, and ensure consistent biological function and manufacturability. It plays a vital role in evaluating clone selection, production process, formulation stability, and batch-to-batch comparability of antibody-based drugs.
Service at MtoZ Biolabs
MtoZ Biolabs offers end-to-end Antibody Drug Glycosylation Characterization Services designed to address the multifaceted analytical needs of glycan analysis across different stages of biopharmaceutical development. Leveraging a comprehensive suite of analytical techniques, we provide in-depth structural and quantitative information for glycans, glycopeptides, and intact glycoproteins. Our platform integrates high-resolution liquid chromatography–mass spectrometry (LC-MS), hydrophilic interaction liquid chromatography coupled with ultra-performance liquid chromatography (HILIC-UPLC), and capillary electrophoresis with laser-induced fluorescence detection (CE-LIF). These methods allow us to deliver precise qualitative and quantitative glycan mapping, site-specific glycosylation analysis, glycoform profiling, and functional interpretation of glycosylation patterns.
Analysis Workflow
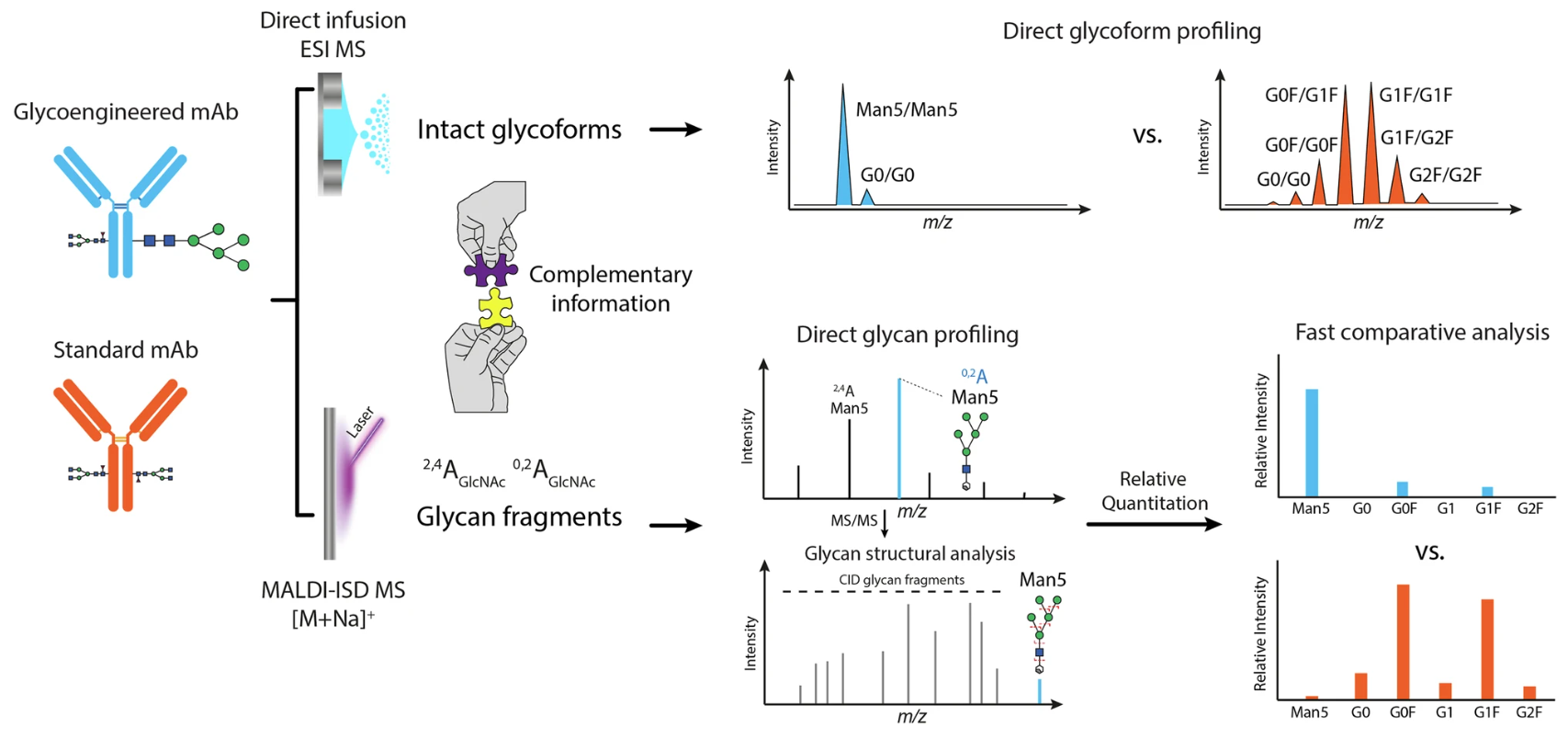
Figure 1. Workflow for Direct Glycosylation Analysis from Intact Standard and Glycoengineered mAbs by ESI MS and MALDI-ISD MS
Why Choose MtoZ Biolabs?
✔️Advanced Analysis Platform
MtoZ Biolabs established an advanced Antibody Drug Glycosylation Characterization Service platform, guaranteeing reliable, fast, and highly accurate analysis service.
✔️High-Data-Quality
Deep data coverage with strict data quality control. AI-powered bioinformatics platform integrates all Antibody Drug Glycosylation Characterization Service data, providing clients with a comprehensive data report.
✔️Comprehensive Service Scope
From released glycan analysis to intact glycoprotein mass profiling, our portfolio covers the full spectrum of antibody glycosylation characterization.
✔️Customized Solutions
We tailor our workflow based on antibody format (IgG, IgA, bispecifics, ADCs), production system, and client objectives.
✔️One-Time-Charge
Our pricing is transparent, no hidden fees or additional costs.
Applications
Antibody glycosylation profiling is vital across a wide spectrum of biopharmaceutical workflows, including:
1. Clone selection and cell line engineering: Evaluate glycan profiles in CHO, HEK293, or glycoengineered cells to select clones that produce desired glycoforms.
2. Quality control and batch release testing: Confirm glycan profile consistency across manufacturing lots to ensure product uniformity.
3. Biosimilar comparability studies: Assess glycosylation similarity between biosimilar and innovator products as part of regulatory submission.
4. Structure-function correlation: Analyze how specific glycan modifications (e.g., afucosylation) impact Fc effector functions such as ADCC.
5. Immunogenicity and safety assessment: Identify non-human or unusual glycans that may trigger immune responses.
6. Stability and degradation analysis: Monitor glycan profile shifts during stress testing and long-term storage.
Sample Submission Suggestions
1. Sample Types
We accept various sample types, including but not limited to:
2. Buffer Requirements
3. Storage and Shipping
*Note: If you have special sample types or require additional guidance, please contact our technical team for personalized support before sample preparation.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Total Ion Chromatogram & Quality Control Assessment (project-dependent)
4. Data Analysis, Preprocessing, and Estimation (project-dependent)
5. Bioinformatics Analysis
6. Raw Data Files
Contact MtoZ Biolabs today to discuss your antibody glycosylation analysis needs and receive a customized consultation and quotation.
Related Services
Glycoprotein Profiling Service
Glycoprotein Structure Analysis Service
How to order?







