DNA/RNA Drug Carrier Characterization Services
- Advanced multi-platform technologies for comprehensive carrier characterization.
- Expertise in DNA/RNA therapeutics, proteomics, and nanomedicine.
- Tailored workflows to meet project-specific requirements and regional regulatory standards.
- High reproducibility and data quality, ensuring reliable results.
- Fast turnaround times with dedicated technical support.
MtoZ Biolabs provides DNA/RNA Drug Carrier Characterization Services to support the development of safe and effective nucleic acid therapeutics. Drug carriers such as lipid nanoparticles (LNPs), virus-like particles (VLPs), polymeric systems, and protein-based carriers play a pivotal role in protecting fragile DNA or RNA molecules, facilitating cellular uptake, and controlling biodistribution. The precise characterization of DNA/RNA drug carriers ensures stability, reproducibility, safety, and efficacy across drug development and manufacturing pipelines.
Drug carriers are structurally complex, often composed of multiple biomolecular or synthetic components. Their functional performance depends on size, charge, morphology, encapsulation efficiency, surface modifications, payload release kinetics, and interactions with nucleic acids. Variability in these parameters can impact therapeutic outcomes, immunogenicity, and regulatory compliance. By characterizing carrier systems at both the physicochemical and functional levels, researchers can optimize formulations, monitor batch-to-batch consistency, and meet stringent quality standards.
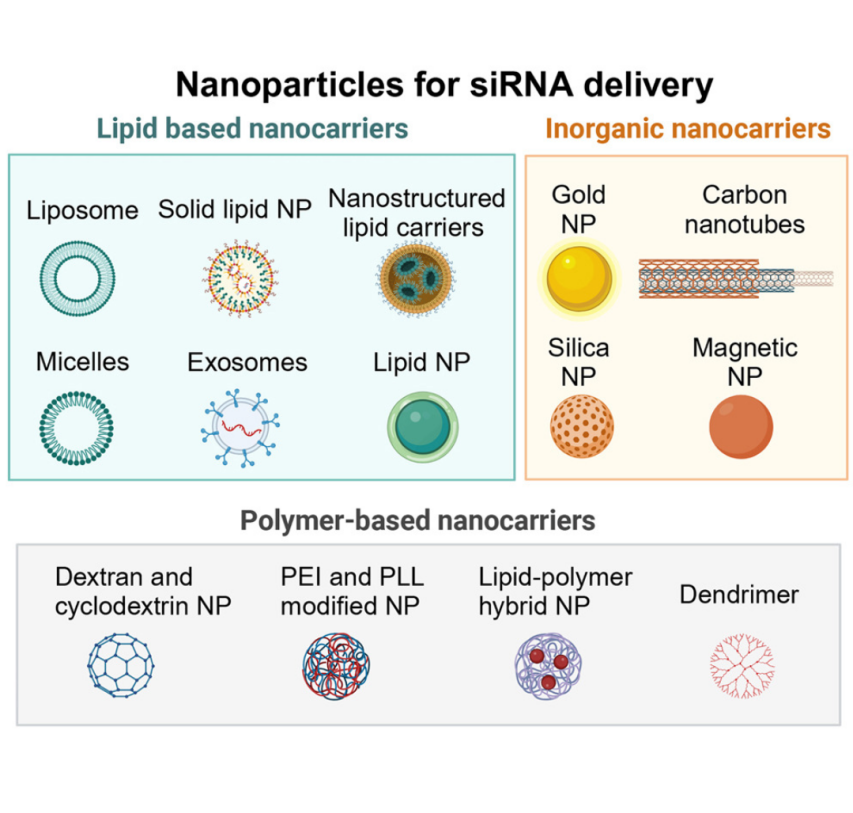
Moazzam, M. et al. Mol Ther. 2024.
Service at MtoZ Biolabs
At MtoZ Biolabs, we deliver comprehensive analytical solutions tailored to DNA and RNA drug carrier systems. Leveraging advanced instrumentation and specialized workflows, we provide insights that span structural, compositional, and functional dimensions of carriers.
Our comprehensive DNA/RNA drug carrier analytical solutions include, but are not limited to:
1. Carrier Capsid Protein Composition Analysis Service
This service focuses on identifying and quantifying the proteins that form carrier capsids, coupled with structural characterization to ensure integrity and consistency in nucleic acid delivery systems.
2. LNP Encapsulation Efficiency Measurement Service
Advanced analytical platforms are applied to measure how effectively DNA or RNA molecules are encapsulated within lipid nanoparticles (LNPs). The results support reproducible formulation and optimization of drug-to-lipid ratios.
3. VLP Encapsulation Efficiency Measurement Service
Through specialized methods, the efficiency of virus-like particles (VLPs) in packaging nucleic acids is assessed, providing essential data on stability, loading capacity, and delivery performance.
4. LNP Composition Analysis Service
Detailed profiling of lipid composition is performed to evaluate the ratio and identity of lipid constituents within LNP formulations. This analysis links composition with stability, performance, and safety outcomes.
Analysis Workflow
Service Advantages
Applications
1. Therapeutic Applications
Cancer immunotherapy, infectious disease treatment, and rare genetic disorder therapy
2. Formulation Development
Optimization of carrier composition, adjustment of encapsulation strategies, and enhancement of delivery efficiency
3. Mechanistic Studies
Understanding carrier–nucleic acid interactions, investigating stability under physiological conditions, and mapping biodistribution pathways
4. Comparative Evaluation
Benchmarking LNPs, VLPs, polymeric, and protein-based carriers for performance and safety
5. Translational Research
Bridging preclinical findings with clinical applications, supporting dose design and delivery route selection
6. Quality Assurance
Consistency monitoring across batches, impurity detection, and long-term stability evaluation
Reliable carrier characterization is essential for advancing DNA and RNA therapeutics. MtoZ Biolabs delivers accurate, efficient, and tailored solutions that help ensure stability, safety, and regulatory compliance. Free project evaluation, welcome to learn more details!
How to order?







