LNP Composition Analysis Service | DNA/RNA Drug
- Detailed description of experimental workflow and methodologies.
- Raw data files available upon request.
- Identification and quantitative data on lipid and nucleic acid composition.
- Impurity and degradation profiles when applicable.
- Stability and batch consistency assessments.
- Data interpretation and a comprehensive final report.
MtoZ Biolabs provides professional LNP Composition Analysis Service to support DNA and RNA drug development. Lipid nanoparticles (LNPs) have become the leading non-viral delivery vehicles for nucleic acid drugs, including mRNA vaccines, siRNA therapies, and gene editing platforms. As nanoscale carriers composed of multiple lipid components, LNPs encapsulate and protect fragile DNA or RNA molecules, enabling efficient delivery to target tissues while minimizing enzymatic degradation.
The composition of LNPs plays a decisive role in their drug-loading capacity, stability, biodistribution, immunogenicity, and therapeutic performance. Small changes in lipid ratios or the presence of impurities can significantly impact encapsulation efficiency, release kinetics, and clinical safety. Comprehensive LNP composition analysis is therefore essential across all stages of drug development, from formulation screening to large-scale manufacturing and regulatory submission.
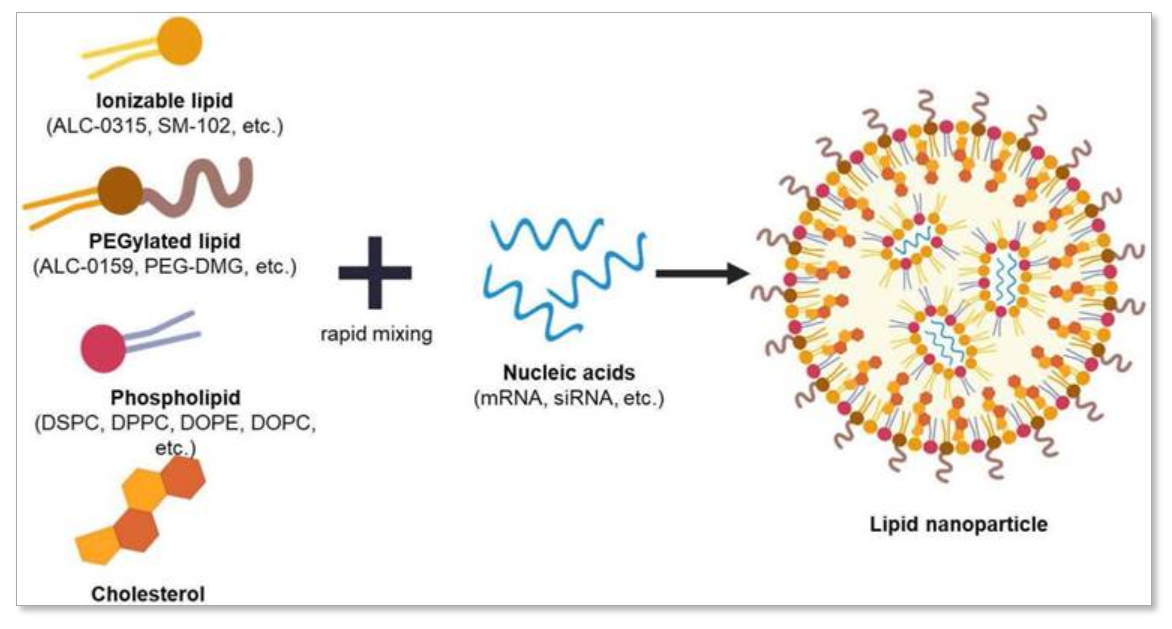
Figure 2. Illustration of Lipid Nanoparticle and Its Compositions
Service at MtoZ Biolabs
Our service characterizes the identity, quantity, and structural role of LNP components while detecting possible degradation or batch variability. With advanced instrumentation and expert workflows, MtoZ Biolabs delivers precise and reliable results that help clients optimize LNP formulations and meet regulatory standards for DNA/RNA-based therapeutics.
💠LNP Characterization
Comprehensive evaluation of physicochemical properties, including particle size, polydispersity index, surface charge (zeta potential), and morphology. These parameters are critical for understanding nanoparticle stability, biodistribution, and delivery efficiency.
💠Lipid Identification and Quantification
Determining the identity and molar ratios of lipid constituents within the nanoparticle, including ionizable lipids, helper lipids, cholesterol, and PEGylated lipids.
💠Encapsulation Efficiency Analysis
Determining the proportion of nucleic acid molecules successfully encapsulated within LNPs compared to the total nucleic acid input. This ensures accurate evaluation of loading efficiency and supports consistent formulation quality.
💠Impurity and Degradation Profiling
Detection of unwanted by-products, oxidized lipids, or other impurities that may affect stability or therapeutic safety.
💠Compositional Stability Studies
Analysis of how storage conditions, stress testing, and time influence the chemical integrity and functional stability of LNPs.
Analysis Workflow
1. Separation and Purification
Isolate and purify lipid nanoparticles from the sample matrix to ensure accurate downstream analysis.
2. Extraction
Extract lipid and nucleic acid components from purified LNPs using optimized protocols to preserve composition integrity.
3. Instrumental Analysis
Perform advanced analytical measurements using techniques such as liquid chromatography, mass spectrometry, nuclear magnetic resonance (NMR) spectroscopy, and UV-Vis spectroscopy to characterize lipid and nucleic acid components.
4. Data Analysis
Process raw data to determine lipid composition, nucleic acid content, and encapsulation efficiency, followed by interpretation and reporting.
Why Choose MtoZ Biolabs?
☑️Proven track record in complex nanoparticle characterization supporting both early-stage research and late-stage development.
☑️Integration of lipidomics and nucleic acid analytics for holistic evaluation of LNP formulations.
☑️Flexible project design allowing seamless adaptation to novel drug delivery platforms.
☑️Rapid turnaround times without compromising data quality or scientific rigor.
☑️Dedicated client support ensuring transparent communication and tailored reporting formats.
Applications
1. Drug Development: Screening and optimizing LNP formulations for DNA/RNA therapeutics.
2. Manufacturing Quality Control: Ensuring reproducibility of lipid ratios and encapsulation efficiency across production batches.
3. Regulatory Submission: Providing compositional data required for demonstrating product quality and safety.
4. Stability Studies: Assessing compositional changes under stress conditions or during long-term storage.
5. Comparative Research: Evaluating LNP performance across different formulations, lipid chemistries, or manufacturing processes.
Deliverables
The professional LNP Composition Analysis Service of MtoZ Biolabs helps DNA and RNA drug developers ensure the quality, stability, and safety of their formulations. Free project evaluation, welcome to learn more details!
Related Services
VLP Encapsulation Efficiency Measurement Service | DNA/RNA Drug
Carrier Capsid Protein Composition Analysis Service | DNA/RNA Drug
How to order?







