LNP Encapsulation Efficiency Measurement Service | DNA/RNA Drug
- DNA and RNA drug formulation development
- Optimization of lipid nanoparticle delivery systems
- Batch-to-batch quality control and consistency monitoring
- Preclinical and clinical research support
- Regulatory submission for nucleic acid therapeutics
Lipid Nanoparticles (LNPs) have become the leading delivery system for nucleic acid drugs, offering protection for fragile DNA and RNA molecules, enhancing cellular uptake, and improving pharmacokinetic properties. MtoZ Biolabs provides professional LNP Encapsulation Efficiency Measurement Service to support pharmaceutical and biotechnology researchers in the development of safe and effective DNA and RNA therapeutics.
LNPs are nanoscale carriers composed of lipids that can encapsulate and protect therapeutic nucleic acids, ensuring their stability and enabling efficient delivery to target cells. The encapsulation efficiency of LNPs refers to the proportion of nucleic acids successfully packaged within the nanoparticles relative to the total nucleic acid input. This metric is crucial for evaluating drug loading capacity, optimizing formulations, ensuring batch-to-batch consistency, and meeting quality and regulatory standards. Accurate measurement of encapsulation efficiency provides insights into the performance of LNP formulations and their suitability for clinical and commercial applications.
Service at MtoZ Biolabs
MtoZ Biolabs' LNP Encapsulation Efficiency Measurement Service ensures accurate evaluation of how effectively therapeutic nucleic acids are packaged within lipid nanoparticles (LNPs), which is essential for stability, delivery efficiency, and regulatory compliance.
We integrate multiple analytical strategies to deliver precise results:
💠LNP Characterization
We examine particle size, polydispersity index, surface charge, and morphology using dynamic light scattering (DLS), zeta potential analysis, and electron microscopy. This step confirms the integrity and uniformity of the nanoparticles before efficiency measurement.
💠Encapsulation Efficiency Measurement
Using advanced techniques such as high-performance liquid chromatography (HPLC), UV-Vis spectroscopy, cryogenic transmission electron microscopy (Cryo-EM), fluorescence-based quantification, and mass spectrometry, we determine the proportion of nucleic acids successfully encapsulated within LNPs, ensuring reliable drug loading assessment.
💠Stability Testing
We evaluate how encapsulated drugs respond to environmental factors such as temperature, pH, and storage conditions, helping clients identify potential risks in formulation and optimize storage protocols.
💠Release Kinetics
Through controlled in vitro studies, we monitor the release profile of nucleic acids from LNPs under physiological conditions, providing insights into drug availability and therapeutic performance.
Analysis Workflow
1. Drug Loading
DNA or RNA therapeutic molecules are incorporated into lipid nanoparticles through carefully optimized formulation procedures, ensuring stable encapsulation.
2. Purification
The encapsulated LNPs are separated from free, unencapsulated nucleic acids using suitable purification strategies, such as ultracentrifugation or size-exclusion chromatography, to obtain clean formulations for analysis.
3. Quantification
High-performance liquid chromatography (HPLC), UV-Vis spectroscopy, or other validated methods are employed to measure the amount of DNA or RNA successfully encapsulated within the nanoparticles.
4. Encapsulation Efficiency Calculation
Encapsulation efficiency (EE%) is calculated based on the ratio of encapsulated nucleic acids to the total nucleic acids used in the formulation, expressed as a percentage:
EE%=(Encapsulated Nucleic Acids/Total Nucleic Acids Used)×100
5. Data Analysis
The experimental results are carefully analyzed to evaluate encapsulation performance and consistency. Based on these findings, recommendations are provided to optimize drug formulations, improve stability, and enhance delivery efficiency.
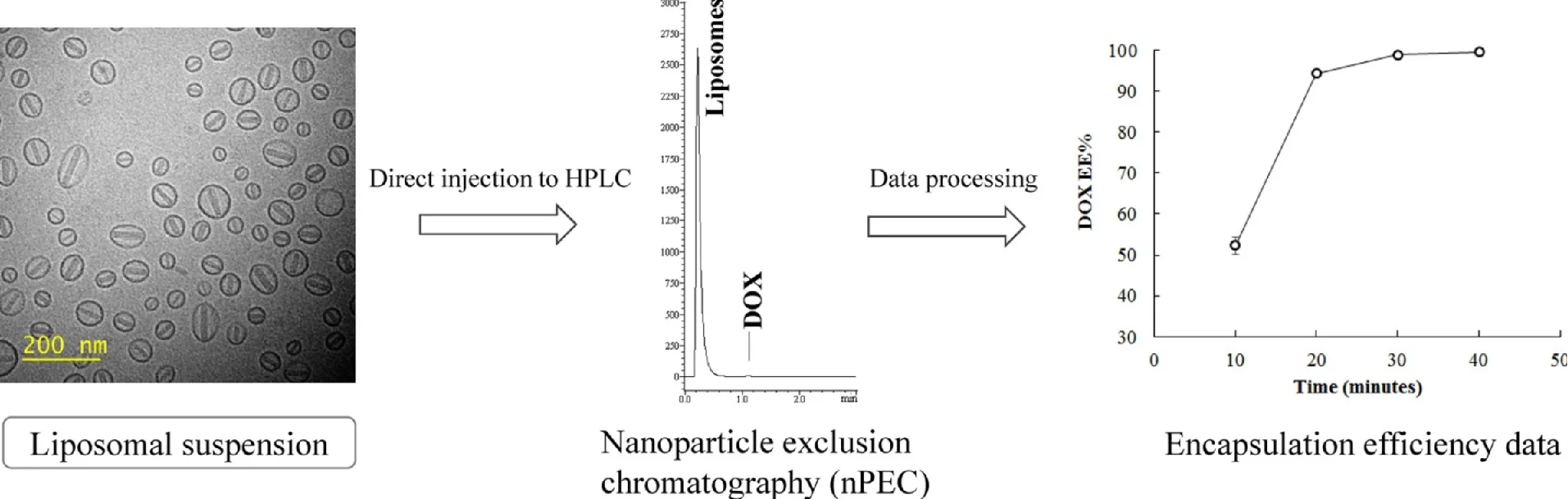
Figure 1. Liposome Encapsulation Efficiency Analysis
Service Advantages
✅An advanced LNP encapsulation efficiency measurement platform integrating chromatography, spectroscopy, mass spectrometry, and other methods for reliable and accurate results.
✅High sensitivity and precision in detecting encapsulated nucleic acids, ensuring meaningful evaluation of drug loading performance.
✅Efficient workflows designed to deliver results within short turnaround times while maintaining high reproducibility.
✅Flexibility to customize testing approaches based on specific drug formulations, project objectives, and regulatory requirements.
✅Expert scientific team with deep experience in nucleic acid drug development and nanoparticle characterization.
Applications
FAQ
Q: Is it possible to assess encapsulation efficiency under simulated physiological conditions?
A: Yes, we can perform encapsulation stability and release studies under conditions mimicking physiological pH, ionic strength, or serum exposure to better predict in vivo performance.
Deliverables
1. Comprehensive experimental details
2. Materials, instruments, and methods
3. Raw data files
4. Encapsulation efficiency data with quantitative results
5. Data interpretation and report
MtoZ Biolabs is committed to providing reliable, comprehensive, and high-quality LNP Encapsulation Efficiency Measurement Services to support DNA/RNA drug development. Free project evaluation, welcome to learn more details!
Related Services
VLP Encapsulation Efficiency Measurement Service | DNA/RNA Drug
Carrier Capsid Protein Composition Analysis Service | DNA/RNA Drug
How to order?







